D84601
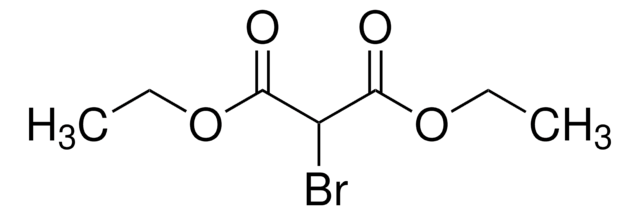
Diethyl acetamidomalonate
98%
Synonym(s):
Acetamidomalonic acid diethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CONHCH(CO2C2H5)2
CAS Number:
Molecular Weight:
217.22
Beilstein:
783883
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
bp
185 °C/20 mmHg (lit.)
mp
95-98 °C (lit.)
SMILES string
CCOC(=O)C(NC(C)=O)C(=O)OCC
InChI
1S/C9H15NO5/c1-4-14-8(12)7(10-6(3)11)9(13)15-5-2/h7H,4-5H2,1-3H3,(H,10,11)
InChI key
ISOLMABRZPQKOV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sambasivarao Kotha et al.
Amino acids, 41(4), 933-936 (2010-11-10)
Strategically a new approach for the synthesis tetrahydro-β-carboline unit with the aid of diethyl acetamidomalonate as a glycine equivalent has been described.
Soraia Veloso Silva Santana et al.
Journal of esthetic and restorative dentistry : official publication of the American Academy of Esthetic Dentistry ... [et al.], 21(6), 397-404 (2009-12-17)
Surface sealants may reduce or avoid problems related to the marginal interface. The aim of this study was to evaluate the microleakage in resin composite Class V restorations sealed with an adhesive system (Xeno III [Dentsply, Konstanz, Germany]), a sealant
G K Powell et al.
Preparative biochemistry, 11(3), 339-350 (1981-01-01)
This paper describes the complete chemical synthesis of 4-methylene-DL-glutamic acid from diethylmalonate, formaldehyde and diethyl acetamidomalonate. The amino acid was obtained pure following ion-exchange chromatography and/or crystallization from hot water in an overall yield of 30% based on the amount
Zsolt Rapi et al.
Carbohydrate research, 365, 61-68 (2012-12-12)
The synthesis of four new ribo-hexopyranoside-based chiral lariat ethers of monoaza-15-crown-5 type and two altropyranoside-based crown ethers were elaborated. Our syntheses utilized the regioselective ring opening of the oxiran moiety of the 2,3-anhydro sugars by nucleophilic reagents to afford the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service