All Photos(1)
About This Item
Empirical Formula (Hill Notation):
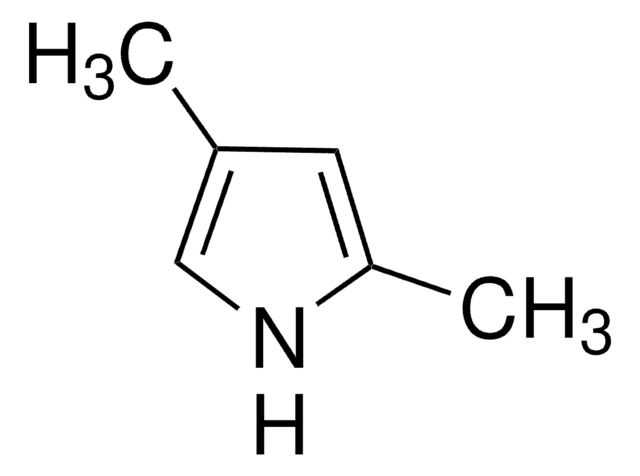
C6H9N
CAS Number:
Molecular Weight:
95.14
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.505 (lit.)
bp
165 °C/740 mmHg (lit.)
density
0.935 g/mL at 25 °C (lit.)
SMILES string
Cc1ccc(C)[nH]1
InChI
1S/C6H9N/c1-5-3-4-6(2)7-5/h3-4,7H,1-2H3
InChI key
PAPNRQCYSFBWDI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
129.2 °F - closed cup
Flash Point(C)
54 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Yoshiteru Matsumoto et al.
Physical chemistry chemical physics : PCCP, 13(31), 13962-13971 (2011-06-16)
N-H···π hydrogen-bonded (H-bonded) structures were studied by applying vibrational spectroscopy to self-aggregate clusters of 2,5-dimethylpyrrole (DMPy) and its binary clusters with pyrrole (Py). The NH stretching vibrations of jet-cooled clusters were observed by IR cavity ringdown spectroscopy. A combination of
M Zhu et al.
Journal of chromatography, 628(1), 37-47 (1993-01-01)
A method employing high-performance liquid chromatography with thermospray mass spectrometry (TSP-MS) and photodiode-array detection was developed and applied to the analysis of autoxidation products of 2,5-dimethyl-N-alkylpyrroles in aqueous solution under air or 18O2. Numerous oxidation products were separated, characterized and
Hongyin Yin et al.
PloS one, 8(9), e76011-e76011 (2013-10-08)
The formation of pyrrole adducts might be responsible for peripheral nerve injury caused by n-hexane. The internal dose of pyrrole adducts would supply more information for the neurotoxicity of n-hexane. The current study was designed to investigate the tissue distributions
Ireneusz Nowak et al.
Organic letters, 5(18), 3345-3348 (2003-08-29)
[reaction: see text] Protection of the amino group of adenine and guanine nucleosides was effected by heating the substrates in 2,5-hexanedione. The resulting 2,5-dimethylpyrrole adducts were stable toward bases but were hydrolyzed with TFA/H(2)O to regenerate the amino function.
Determination of urinary 2,5-hexanedione by its conversion to 2,5-dimethylpyrrole.
M Ogata et al.
Industrial health, 28(3), 125-131 (1990-01-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service