519235
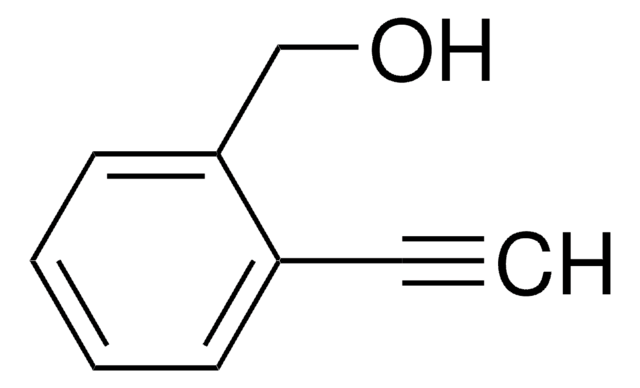
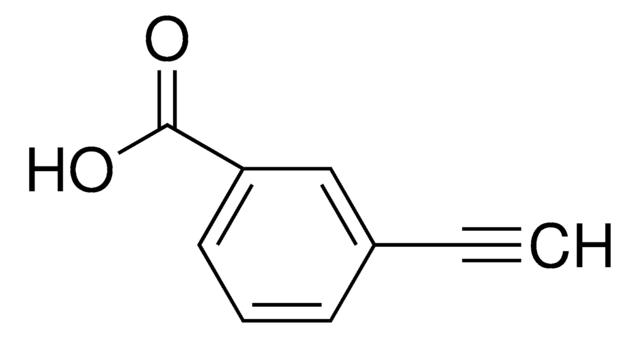
4-Ethynylbenzyl alcohol
97%
Synonym(s):
4-Hydroxymethylphenylacetylene
About This Item
Recommended Products
Quality Level
Assay
97%
mp
40-44 °C (lit.)
functional group
hydroxyl
SMILES string
OCc1ccc(cc1)C#C
InChI
1S/C9H8O/c1-2-8-3-5-9(7-10)6-4-8/h1,3-6,10H,7H2
InChI key
QCZORVSTESPHCO-UHFFFAOYSA-N
Application
- 4-Ethynylbenzyl alcohol can be used in the synthesis of platinum-acetylide dendrimers, rotaxanes and arylacetylenes.
- It was employed in the preparation of arylalkyne-tagged sugars for the photoinduced glycosylation of cysteine-containing peptides.
- It can also act as a precursor to synthesize fluorescent probes via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) click reaction.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service