391794
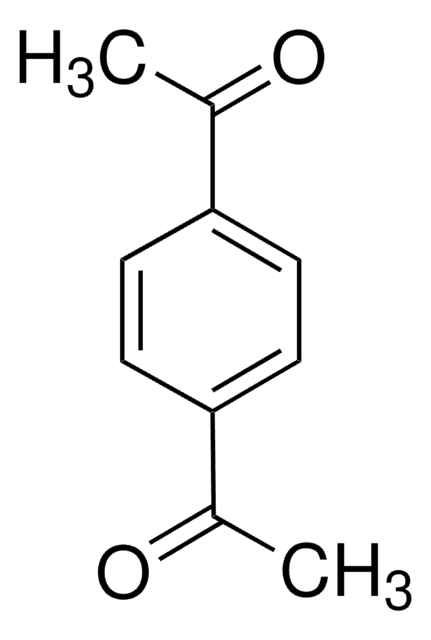
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone
99%
Synonym(s):
4,6-Diacetylresorcinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(HO)2C6H2(COCH3)2
CAS Number:
Molecular Weight:
194.18
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
178-180 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1cc(C(C)=O)c(O)cc1O
InChI
1S/C10H10O4/c1-5(11)7-3-8(6(2)12)10(14)4-9(7)13/h3-4,13-14H,1-2H3
InChI key
GEYCQLIOGQPPFM-UHFFFAOYSA-N
Related Categories
General description
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) is a bifunctional carbonyl compound. Its synthesis by acetylating resorcinol in the presence of zinc chloride has been reported. The crystal structure of DAR has been studied.
Application
1,1′-(4,6-Dihydroxy-1,3-phenylene)bisethanone (4,6-diacetylresorcinol, DAR) may be used in the synthesis of the following:
- Schiff base ligands
- hexadentate chalcogenated bisimine ligands
- 1,5-benzodiazepines
- ketimine of chitosan
- mannich bases
- hydrazone ligands
- thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands
- binuclear cobalt(II) and copper(II) complexes
- europium (III) complexes
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Shebl et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(1), 428-436 (2009-12-08)
Mono- and binuclear VO(IV), Ce(III), Th(IV) and UO(2)(VI) complexes of thiosemicarbazone, semicarbazone and thiocarbohydrazone ligands derived from 4,6-diacetylresorcinol were synthesized. The structures of these complexes were elucidated by elemental analyses, IR, UV-vis, ESR, (1)H NMR and mass spectra as well
Structure of 4, 6-diacetylresorcinol.
Kokila MK, et al.
Acta Crystallographica Section C, Crystal Structure Communications, 48(6), 1133-1134 (1992)
Cahit Demetgül
Carbohydrate polymers, 89(2), 354-361 (2012-06-20)
In this study, a new chitosan derivative (ketimine) was synthesized by condensation of chitosan with 4,6-diacetylresorcinol (DAR) at heterogeneous medium. The ketimine derivative of chitosan (DAR-chitosan) was characterized by elemental (C, H, N), spectral (DR-UV-vis and FT-IR spectroscopy), structural (powder
A Facile Synthesis of 2-Benzoyl-6-Hydroxy-3-Methyl-5-(2-Substituted-2, 3-Dihydro-1H-1,5-Benzodiazepin-4-YL) Benzo [b] Furans.
Reddy K, et al.
Synthetic Communications, 30(10), 1825-1836 (2000)
Arun Kumar et al.
Journal of hazardous materials, 269, 9-17 (2013-12-10)
Potentially hexadentante [O(-),N,E:E,N,O(-)] chalcogenated bisimine ligands L1-L3 have been synthesized by reaction of 1,1'-(4,6-dihydroxy-1,3-phenylene)bisethanone with H2N(CH2)2SPh, H2N(CH2)2SePh and H2N(CH2)2TeC6H4-4-OMe respectively. The L1-L3 react with Na2PdCl4 resulting in their partial hydrolysis, which appears to be metal-promoted. Of the two [(CH3)CN(CH2)2EAr] fragments
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service