133302
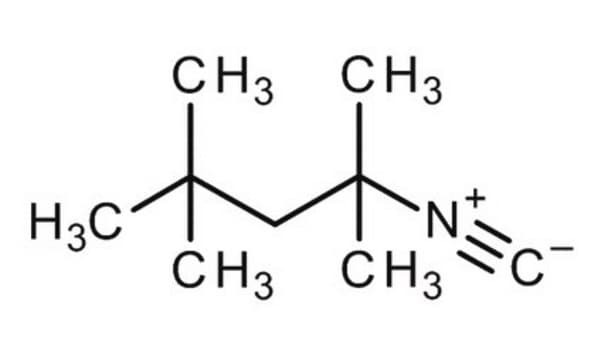
Cyclohexyl isocyanide
98%
Synonym(s):
Isocyanocyclohexane
About This Item
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.45 (lit.)
density
0.878 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[C-]#[N+]C1CCCCC1
InChI
1S/C7H11N/c1-8-7-5-3-2-4-6-7/h7H,2-6H2
InChI key
XYZMOVWWVXBHDP-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Isocyanide in coordination chemistry: The study on mixed "2 + 1" tricarbonyl dithiocarbamate complexes highlights Cyclohexyl isocyanide′s role as an effective monodentate ligand, contributing to advancements in radiopharmaceutical applications using Re, Tc, and Re isotopes (Shegani et al., 2021).
- Organic synthesis reagent in vascular treatments: The article discusses the use of Cyclohexyl isocyanide in modifying the endocannabinoid system, emphasizing its potential in developing treatments for conditions like hypertension through biochemical pathway modulation (Baranowska-Kuczko et al., 2021).
- Chemical process optimization in dye decolorization: Cyclohexyl isocyanide plays a crucial role in the covalent immobilization of enzymes used for the decolorization of textile dyes, demonstrating its utility in environmental chemistry and industrial applications related to pollution control (Salami et al., 2018).
- Application in nanocellulose modification: Demonstrates the versatility of Cyclohexyl isocyanide in nanotechnology by facilitating the covalent attachment of temperature-responsive polymers to cellulose nanofibrils, enhancing the material′s properties for use in smart textiles and responsive materials (Khine et al., 2018).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
170.6 °F - closed cup
Flash Point(C)
77 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service