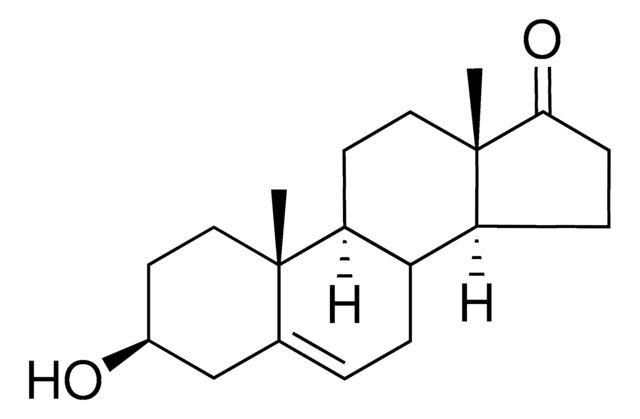
H5002
17α-Hydroxypregnenolone
Synonym(s):
3β,17α-Dihydroxy-5-pregnen-20-one, 5-Pregnene-3β,17α-diol-20-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C21H32O3
CAS Number:
Molecular Weight:
332.48
EC Number:
MDL number:
UNSPSC Code:
41116107
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
SMILES string
C[C@]1(CC[C@H](O)C2)C2=CC[C@]3([H])[C@]1([H])CC[C@@]4(C)[C@@]3([H])CC[C@@]4(C(C)=O)O
InChI
1S/C21H32O3/c1-13(22)21(24)11-8-18-16-5-4-14-12-15(23)6-9-19(14,2)17(16)7-10-20(18,21)3/h4,15-18,23-24H,5-12H2,1-3H3
InChI key
JERGUCIJOXJXHF-UHFFFAOYSA-N
General description
17α-hydroxypregnenolone is a derived from pregnenolone.
Application
17α-hydroxypregnenolone has been used as a substrate for the enzyme 3β‐hydroxysteroid dehydrogenase (3β‐HSD) expressed in COS1 cells.
Biochem/physiol Actions
17α-hydroxypregnenolone acts as a precursor for cortisol and sex steroids.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
[17alpha-Hydroxypregnenolone].
Yukiko Shimizu
Nihon rinsho. Japanese journal of clinical medicine, 68 Suppl 7, 367-370 (2010-10-22)
Tamara S Hannon et al.
Journal of pediatric and adolescent gynecology, 25(1), 82-85 (2011-11-18)
Little is known about racial differences in androgen levels among obese children. The objective of this pilot study was to compare basal and stimulated androgen levels in a cross-sectional sample of obese black and white pubertal females. STUDY DESIGN, SETTING
Shogo Haraguchi et al.
Endocrinology, 153(2), 794-805 (2011-12-01)
7α-Hydroxypregnenolone (7α-OH PREG) is a newly identified bioactive neurosteroid stimulating locomotor activity in the brain of newt, a wild animal, which serves as an excellent model to investigate the biosynthesis and biological action of neurosteroids. Here, we show that acute
Two novel HSD3B2 missense mutations with diverse residual enzymatic activities for Delta 5-steroids
TakasawaK, et al.
Clin. Endocrinol., 80(6), 782-789 (2014)
Sung Mee Kim et al.
Clinical and experimental reproductive medicine, 42(2), 72-76 (2015-07-15)
17α-hydroxylase and 17,20-lyase are enzymes encoded by the CYP17A1 gene and are required for the synthesis of sex steroids and cortisol. In 17α-hydroxylase deficiency, there are low blood levels of estrogens, androgens, and cortisol, and resultant compensatory increases in adrenocorticotrophic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service