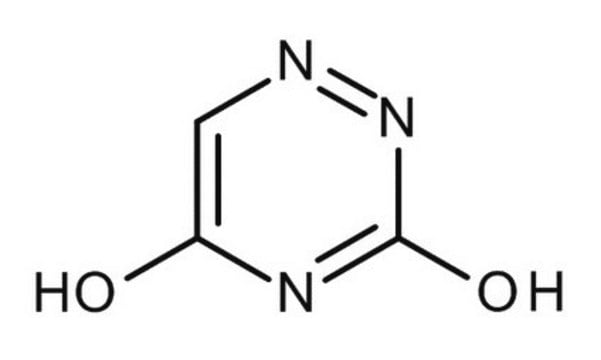
A1757
6-Azauracil
≥98%
Synonym(s):
6-AU, 1,2,4-Triazine-3,5(2H,4H)-dione, 3,5-Dihydroxy-1,2,4-triazine, 6-Aza-2,4-dihydroxypyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C3H3N3O2
CAS Number:
Molecular Weight:
113.07
Beilstein:
116472
EC Number:
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.51
Recommended Products
Quality Level
Assay
≥98%
form
powder
mp
274-275 °C (lit.)
solubility
1 M NH4OH: 50 mg/mL, clear to slightly hazy, colorless to light yellow-green
SMILES string
O=C1NN=CC(=O)N1
InChI
1S/C3H3N3O2/c7-2-1-4-6-3(8)5-2/h1H,(H2,5,6,7,8)
InChI key
SSPYSWLZOPCOLO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
6-Azauracil has been used as a transcriptional inhibitor to study its effects on the deletion of termination and polyadenylation protein (Tpa1) and Mag1 on cell viability. It has also been used as an orotidine-5′-monophosphate decarboxylase (OMPdecase) inhibitor in minimal media for determining the OMPdecase activity.
Biochem/physiol Actions
6-Azauracil (6-AU) is a pyrimidine analog of uracil and exhibits antitumor activity. It inhibits the growth of various microorganisms by depleting intracellular guanosine triphosphate (GTP) and uridine triphosphate (UTP) nucleotide pools.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mihajlo Etinski et al.
Physical chemistry chemical physics : PCCP, 12(48), 15665-15671 (2010-08-04)
The photophysical properties of 6-azauracil were studied by means of ab initio quantum chemical methods. On the basis of our calculations we propose here the following mechanism for the lack of fluorescence and the high triplet quantum yield that was
methods in molecular biology
steroid receptor methods (2001)
Sittinan Chanarat et al.
Genes & development, 25(11), 1147-1158 (2011-05-18)
Different steps in gene expression are intimately linked. In Saccharomyces cerevisiae, the conserved TREX complex couples transcription to nuclear messenger RNA (mRNA) export. However, it is unknown how TREX is recruited to actively transcribed genes. Here, we show that the
Allan Guiguen et al.
The EMBO journal, 26(6), 1552-1559 (2007-03-03)
Capping of nascent pre-mRNAs is thought to be a prerequisite for productive elongation and associated serine 2 phosphorylation of the C-terminal domain (CTD) of RNA polymerase II (PolII). The mechanism mediating this link is unknown, but is likely to include
Mattias Carlsson et al.
PloS one, 13(5), e0196840-e0196840 (2018-05-09)
Purine and pyrimidine analogues have important uses in chemotherapies against cancer, and a better understanding of the mechanisms that cause resistance to these drugs is therefore of importance in cancer treatment. In the yeast Saccharomyces cerevisiae, overexpression of the HAM1
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service