All Photos(1)
About This Item
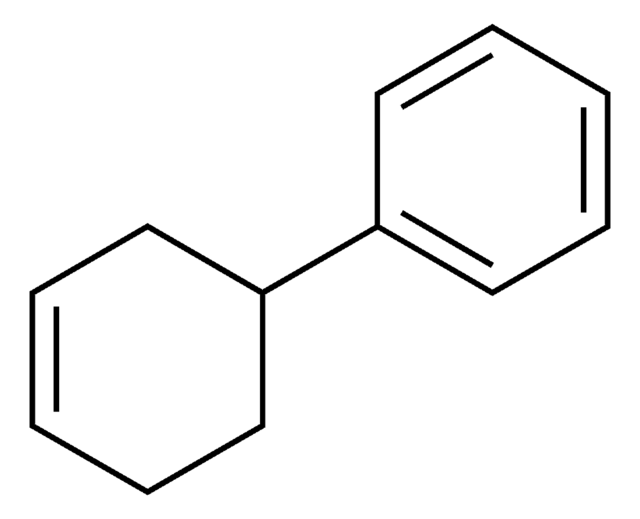
Linear Formula:
C6H5C6H9
CAS Number:
Molecular Weight:
158.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.57 (lit.)
bp
251-253 °C (lit.)
mp
−11 °C (lit.)
density
0.994 g/mL at 25 °C (lit.)
SMILES string
C1CCC(=CC1)c2ccccc2
InChI
1S/C12H14/c1-3-7-11(8-4-1)12-9-5-2-6-10-12/h1,3-4,7-9H,2,5-6,10H2
InChI key
WCMSFBRREKZZFL-UHFFFAOYSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
217.4 °F - closed cup
Flash Point(C)
103.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A S Freeman et al.
Drug metabolism and disposition: the biological fate of chemicals, 10(6), 680-684 (1982-11-01)
Mice were exposed to the smoke from placebo marihuana cigarettes treated with phencyclidine hydrochloride (PCP . HCl). A dose-related decrement in motor performance was observed after exposure to the smoke from cigarettes containing 10-15 mg of PCP . HCl. Tissue
C E Cook et al.
Drug metabolism and disposition: the biological fate of chemicals, 12(2), 186-192 (1984-03-01)
In vitro metabolites of 1-phenylcyclohexene produced by the 10,000g supernatant fraction from rat liver homogenates were identified by a combination of spectrometric, chromatographic, and synthetic techniques. Initial oxidation occurred in the 3-position of 1-phenylcyclohexene to yield 1-phenyl-1-cyclohexen-3-one and 1-phenyl-1-cyclohexen-3-ol. Further
F C Law et al.
Drug and chemical toxicology, 7(3), 273-282 (1984-01-01)
The biliary excretion of 14C-phenylcyclohexene and its metabolites were studied in rats pretreated with an inducer or inhibitor of mixed-function oxidases or with an agent known to deplete liver glutathione. Pretreatment of rats with 3-methylcholanthrene or phenobarbital enhanced the biliary
D H Young et al.
Bioorganic & medicinal chemistry letters, 11(11), 1393-1396 (2001-05-30)
Phenylcyclohexenes (PCHs) [e.g., trans-4-nitro-5-(2,3,4-trimethoxyphenyl)cyclohexene, 2d] were found to bind weakly to the colchicine site of bovine tubulin, but are the first mimics of colchicine found to have high activity towards plant cells. Structure-activity relationships for PCHs and biphenyl AC-ring analogues
B R Martin et al.
Drug metabolism and disposition: the biological fate of chemicals, 10(6), 685-689 (1982-11-01)
The in vitro metabolism of 1-3H-phenyl-1-cyclohexene (3H-PC) was studied in a crude microsomal preparation from mouse livers. The major routes of metabolism were allylic hydroxylation, oxidation of the allylic alcohol, and epoxidation-hydrolysis. The following metabolites were identified by comparison with
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service