All Photos(1)
About This Item
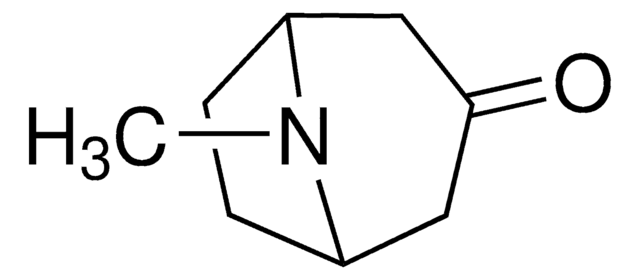
Empirical Formula (Hill Notation):
C8H15NO
CAS Number:
Molecular Weight:
141.21
Beilstein:
80188
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (NT)
form
powder
impurities
0-3% water
solubility
H2O: 0.1 g/mL, clear
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
CN1[C@H]2CC[C@@H]1C[C@H](O)C2
InChI
1S/C8H15NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-8,10H,2-5H2,1H3/t6-,7+,8+
InChI key
CYHOMWAPJJPNMW-JIGDXULJSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Renata A Kwiecień et al.
Archives of biochemistry and biophysics, 510(1), 35-41 (2011-03-23)
(15)N heavy isotope effects are especially useful when detail is sought pertaining to the reaction mechanism for the cleavage of a C-N bond. Their potential in assisting to describe the mechanism of N-demethylation of tertiary amines by the action of
Joshua G Schier et al.
Academic emergency medicine : official journal of the Society for Academic Emergency Medicine, 11(4), 329-334 (2004-04-06)
A massive nerve agent attack may rapidly deplete in-date supplies of atropine. The authors considered using atropine beyond its labeled shelf life. The objective was to determine the stability of premixed injectable atropine sulfate samples with different expiration dates. This
Laszlo Gyermek et al.
Journal of critical care, 24(1), 58-65 (2009-03-11)
There is a need for neuromuscular relaxant (NMR) agents that are of the "nondepolarizing type" and produce rapidly developing and short-lasting skeletal muscle relaxation in anesthesiology. Many efforts have been directed to produce such agents. Our research focused on the
Heike Kaiser et al.
Planta, 225(1), 127-137 (2006-07-18)
Tropinone reductases (TRs) are essential enzymes in the tropane alkaloid biosynthesis, providing either tropine for hyoscyamine and scopolamine formation or providing pseudotropine for calystegines. Two cDNAs coding for TRs were isolated from potato (Solanum tuberosum L.) tuber sprouts and expressed
Amit K Kushwaha et al.
Gene, 516(2), 238-247 (2012-12-26)
Tropinone reductases (TRs) are small proteins belonging to the SDR (short chain dehydrogenase/reductase) family of enzymes. TR-I and TR-II catalyze the conversion of tropinone into tropane alcohols (tropine and pseudotropine, respectively). The steps are intermediary enroute to biosynthesis of tropane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service