723622
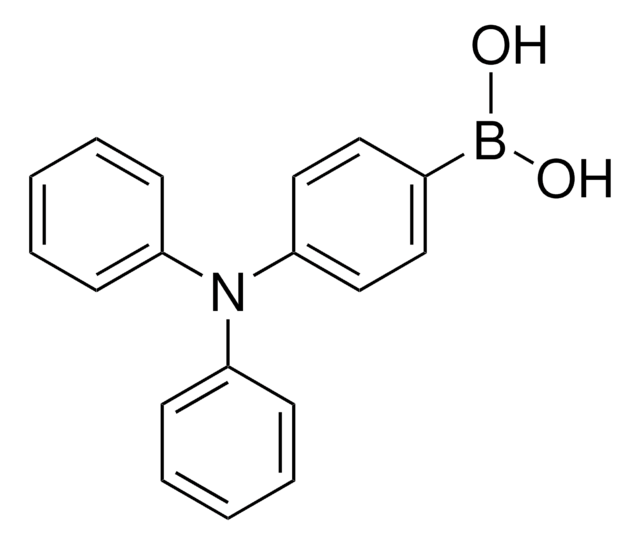
9H-Carbazole-9-(4-phenyl) boronic acid pinacol ester
95%
Synonym(s):
9-(4-(4,4,5,5-Tetramethy-1,3,2-dioxaborolan-2-yl)phenyl)-9H-carbazole
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C24H24BNO2
Molecular Weight:
369.26
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
95%
form
solid
mp
165-173 °C
SMILES string
CC1(C)OB(OC1(C)C)c2ccc(cc2)-n3c4ccccc4c5ccccc35
InChI
1S/C24H24BNO2/c1-23(2)24(3,4)28-25(27-23)17-13-15-18(16-14-17)26-21-11-7-5-9-19(21)20-10-6-8-12-22(20)26/h5-16H,1-4H3
InChI key
AHDSYMVAUJZCOP-UHFFFAOYSA-N
General description
9H-Carbazole-9-(4-phenyl) boronic acid pinacol ester is a compound that is majorly used as an intermediate in electronic devices. Its molecular structure includes benzene rings, boronic acid pinacol ester and carbazole rings.
Application
9H-Carbazole-9-(4-phenyl) boronic acid pinacol can be used as a donor molecule in the formation of novel donor-π-acceptor dyes for electrochemical applications. It can also be used in the formation of organic light emitting diodes (OLEDs).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Spectroscopic studies on 9H-carbazole-9-(4-phenyl) boronic acid pinacol ester by DFT method
Sas EB, et al.
Journal of Molecular Structure, 1118, 124-138 (2016)
Synthesis, photophysical and redox properties of the D-pi-A type pyrimidine dyes bearing the 9-phenyl-9H-carbazole moiety
Verbitskiy EV, et al.
Journal of Fluorescence, 25(3), 763-775 (2015)
New V-shaped 2, 4-di (hetero) arylpyrimidine push-pull systems: Synthesis, solvatochromism and sensitivity towards nitroaromatic compounds
Verbitskiy EV, et al.
Dyes and Pigments, 159(3), 35-44 (2018)
Theoretical and experimental study on spectra, electronic structure and photoelectric properties of three nature dyes used for solar cells
Ren P, et al.
Journal of Molecular Liquids, 247(3), 193-206 (2017)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[9-(4-Bromophenyl)]-3,6-di-tert-butyl-9H-carbazole](/deepweb/assets/sigmaaldrich/product/structures/214/779/819c00e2-ee0a-4166-977c-a7f68002b43d/640/819c00e2-ee0a-4166-977c-a7f68002b43d.png)






