All Photos(1)
About This Item
Empirical Formula (Hill Notation):
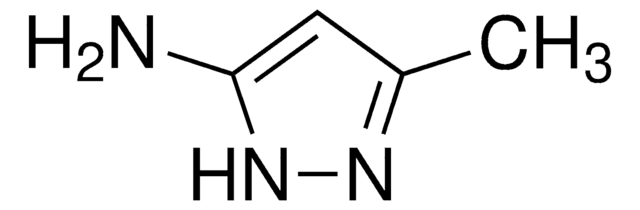
C5H9N3
CAS Number:
Molecular Weight:
111.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
65-69 °C (lit.)
SMILES string
Cc1cc(N)n(C)n1
InChI
1S/C5H9N3/c1-4-3-5(6)8(2)7-4/h3H,6H2,1-2H3
InChI key
ZFDGMMZLXSFNFU-UHFFFAOYSA-N
General description
5-Amino-1,3-dimethylpyrazole undergoes cyclocondensation with ethyl acetoacetate to form the corresponding tetrahydropyrazolopyridine derivatives.
Application
5-Amino-1,3-dimethylpyrazole may be used in the preparation of:
- 5-benzamido-1,3-dimethylpyrazole
- diethyl 2-{[(1,3-dimethyl-1H-pyrazol-5-yl)amino]methylene}malonate
- 5-amino-1,3-dimethyl-4-phthalidylpyrazole
- (E)-N-(3,7-dimethylocta-2,6-dienyl)-1,3-dimethyl-1H-pyrazol-5-amine analog(LQFM002)
- 4-isopropyl-1,3-dimethyl-1H-pyrazolo[3,4-b]pyridin-6-ol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
190.4 °F - closed cup
Flash Point(C)
88 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
The reaction of o?phthalaldchydic acid with 5?amino?1,3?dimethylpyrazole.
Swett LR and Aynilian GH.
Journal of Heterocyclic Chemistry, 12(6), 1135-1136 (1975)
1,3-Oxazines and related compounds. II.1) Ring contraction reaction of 1, 3-oxazin-4-one derivatives into 1,2,4-triazoles and pyrazoles.
Yamamoto, et al.
Chemical & Pharmaceutical Bulletin, 26(6), 1825-1831 (1978)
E A Costa et al.
Life sciences, 92(3), 237-244 (2013-01-09)
The current study describes the synthesis and pharmacological evaluation of (E)-N-(3,7-dimethylocta-2,6-dienyl)-1,3-dimethyl-1H-pyrazol-5-amine (LQFM002), a compound originally designed through a molecular simplification strategy from 4-nerolidylcatechol. LQFM002 was evaluated for preservation of the PLA(2) enzyme inhibitory effects of the lead compound, 4-nerolidylcatechol, using
The cyclocondensation of 5?amino?1,3?dimethylpyrazole with ethyl acetoacetate. Synthesis of isomeric pyrazolopyridones.
Ratajczyk JD and Swett LR.
Journal of Heterocyclic Chemistry, 12(3), 517-522 (1975)
Hiroshi Ochiai et al.
Chemical & pharmaceutical bulletin, 52(9), 1098-1104 (2004-09-02)
A series of 4-anilinopyrazolopyridine derivatives were synthesized and biologically evaluated as inhibitors of phosphodiesterase (PDE4). Chemical modification of 3, a structurally new chemical lead that was found in our in-house library, was focused on 1- and 3-substituents. Full details of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service