All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H8N4O2
CAS Number:
Molecular Weight:
156.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
123-127 °C (lit.)
SMILES string
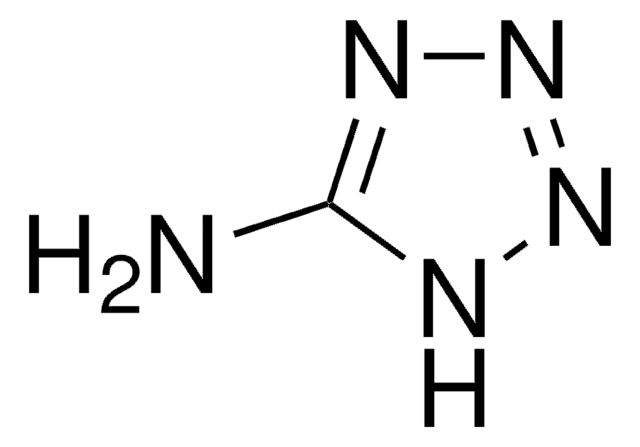
CCOC(=O)Cc1nnn[nH]1
InChI
1S/C5H8N4O2/c1-2-11-5(10)3-4-6-8-9-7-4/h2-3H2,1H3,(H,6,7,8,9)
InChI key
NAOHMNNTUFFTBF-UHFFFAOYSA-N
General description
Ethyl 1H-tetrazole-5-acetate is a tetrazole derivative. Its effect on glutaminyl cyclase activity of the recombinant human glutaminyl cyclase (QC) has been reported.
Application
Ethyl 1H-tetrazole-5-acetate (Ethyl 1H-tetrazole-5-yl acetate, Hetza) may be employed as starting reagent for the synthesis of ethyl aryloxadiazolylacetates. It may be used for the preparation of homochiral 3D diamondoid metal-organic framework [Zn2(etza)4].
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 5-aryl-1, 3, 4-oxadiazolyl-2-acetic acids.
Janda L.
Heterocyclic Communications, 7(5), 411-416 (2001)
A spontaneously resoluted zinc-organic framework with nonlinear optical and ferroelectric properties generated from tetrazolate-ethyl ester ligand.
Liang X-Q, et al.
CrystEngComm, 12(11), 3499-3501 (2010)
Kai-Fa Huang et al.
Protein expression and purification, 43(1), 65-72 (2005-08-09)
Glutaminyl cyclase (QC) catalyzes the N-terminal pyroglutamate formation of numerous hormones and peptides from their glutaminyl precursor. Pyroglutamate is a posttranslational or cotranslational modification important in many physiological and pathological processes. Here, we present the cloning of a QC cDNA
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service