414336
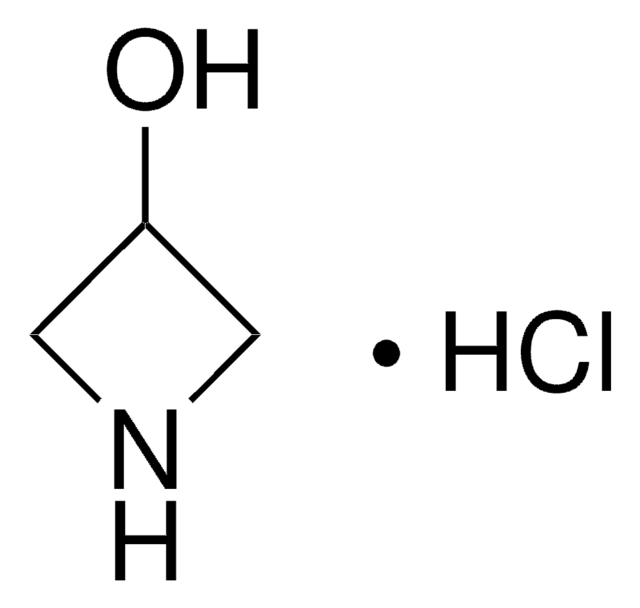
Azetidine hydrochloride
97%
Synonym(s):
Trimethyleneimine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H7N · HCl
CAS Number:
Molecular Weight:
93.56
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
>300 °C (lit.)
SMILES string
Cl[H].C1CNC1
InChI
1S/C3H7N.ClH/c1-2-4-3-1;/h4H,1-3H2;1H
InChI key
HGQULGDOROIPJN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
305.6 °F
Flash Point(C)
152 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Christopher S Dunkley et al.
Bioorganic & medicinal chemistry letters, 13(17), 2899-2901 (2003-11-13)
A series of compounds containing an N-(4'-substituted-3'-nitrophenyl)sydnone moiety with potential antitumor activity was prepared based on active analogues. The rationale behind the design of these compounds is presented along with the 4-step synthetic route to the derivatives in the 4'-position
Valérian Gobé et al.
Organic letters, 16(20), 5438-5441 (2014-10-01)
1,5-/1,6-Allenals conjugated to an aromatic ring undergo a cyclization, in the presence of an amine, that leads to tricyclic compounds including the 1-aminotetralin scaffold. This domino process combines the in situ formation of the enamine and the cyclization affording the
Dongliang Chang et al.
Organic letters, 4(11), 1859-1862 (2002-05-25)
[reaction: see text] Hydroxylation of N-substituted azetidines 11 and 12 and piperidines 15-19 with Sphingomonas sp. HXN-200 gave 91-98% of the corresponding 3-hydroxyazetidines 13 and 14 and 4-hydroxypiperidines 20-24, respectively, with high activity and excellent regioselectivity. High yields and high
Synthesis of ornithine lactams via diastereoselective photocyclization of 2-amino-4-oxo-4-phenyl-butanoyl amines.
Lindemann U, et al.
Tetrahedron Asymmetry, 9(24), 4459-4473 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service