287784
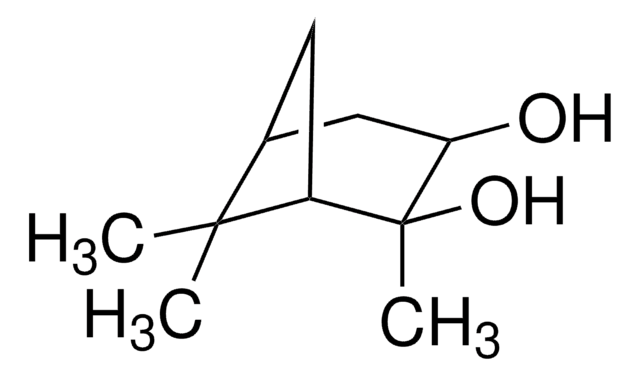
(1R,2R,3S,5R)-(−)-Pinanediol
99%
Synonym(s):
(−)-2-Hydroxyisopinocampheol, [1R-(1α,2α,3α,5α)]-2,6,6-Trimethylbicyclo[3.1.1]heptane-2,3-diol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H18O2
CAS Number:
Molecular Weight:
170.25
Beilstein:
2039144
MDL number:
UNSPSC Code:
12352001
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
optical activity
[α]21/D −8.6°, c = 6.5 in toluene
optical purity
ee: 97% (GLC)
bp
101-102 °C/1 mmHg (lit.)
mp
57-59 °C (lit.)
SMILES string
CC1(C)[C@H]2C[C@H](O)[C@](C)(O)[C@@H]1C2
InChI
1S/C10H18O2/c1-9(2)6-4-7(9)10(3,12)8(11)5-6/h6-8,11-12H,4-5H2,1-3H3/t6-,7-,8+,10-/m1/s1
InChI key
MOILFCKRQFQVFS-BDNRQGISSA-N
Application
Used to prepare chiral double allylation reagents.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Simple, stable, and versatile double-allylation reagents for the stereoselective preparation of skeletally diverse compounds.
Feng Peng et al.
Journal of the American Chemical Society, 129(11), 3070-3071 (2007-02-24)
Gerald I Richard et al.
Applied spectroscopy, 62(5), 476-480 (2008-05-24)
The spectroscopic properties of a chiral boronic acid based resorcinarene macrocycle employed for chiral analysis were investigated. Specifically, the emission and excitation characteristics of tetraarylboronate resorcinarene macrocycle (TBRM) and its quantum yield were evaluated. The chiral selector TBRM was investigated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service