252301
8-Bromo-1-octene
97%
Synonym(s):
1-Bromo-7-octene, 7-Octen-1-yl bromide, 7-Octenyl bromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
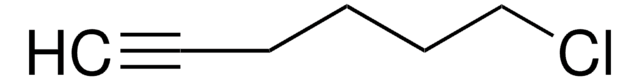
Br(CH2)6CH=CH2
CAS Number:
Molecular Weight:
191.11
Beilstein:
2234481
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.467 (lit.)
density
1.139 g/mL at 25 °C (lit.)
functional group
alkyl halide
allyl
bromo
SMILES string
BrCCCCCCC=C
InChI
1S/C8H15Br/c1-2-3-4-5-6-7-8-9/h2H,1,3-8H2
InChI key
SNMOMUYLFLGQQS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
8-Bromo-1-octene has been used in preparation of polymerizable ligand, required for the synthesis of quantum dot-labelled polymer beads. Grignard reagent derived from 8-bromo-1-octene has been used in the synthesis of (2S,3S,5R)-5-[(1R)-1-hydroxy-9-decenyl]-2-pentyltetrahydro-3-furanol. It has been used as building block in natural product synthesis.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
172.4 °F - closed cup
Flash Point(C)
78 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Paul O'Brien et al.
Chemical communications (Cambridge, England), (20)(20), 2532-2533 (2003-11-05)
CdSe quantum dots with polymerisable ligands have been incorporated into polystyrene beads, via a suspension polymerisation reaction, as a first step towards the optical encoding of solid supports for application in solid phase organic chemistry.
Enantiocontrolled synthesis of C-19 tetrahydrofurans isolated from the marine alga Notheia anomala.
Garci'a C, et al.
Tetrahedron Letters, 41(21), 4127-4130 (2000)
Christophe Dubost et al.
Organic letters, 8(22), 5137-5140 (2006-10-20)
The total synthesis of the polyhydroxylated macrolide (+)-aspicilin 5 is described using as a key step a highly diastereoselective allylation of aldehyde 6 with the uniquely functionalized allylstannane 1. (+)-Aspicilin is obtained in 18 steps and 10% overall yield. [structure:
Carolyn A Leverett et al.
The Journal of organic chemistry, 71(22), 8591-8601 (2006-10-27)
cis-2-Methyl-6-substituted piperidin-3-ol alkaloids of the Cassia and Prosopis species are readily prepared by a combination of an aza-Achmatowicz oxidative rearrangement and dihydropyridone reduction followed by a stereoselective allylsilane addition to a N-sulfonyliminium ion. The stereochemical outcome of the reduction reaction
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service