237876
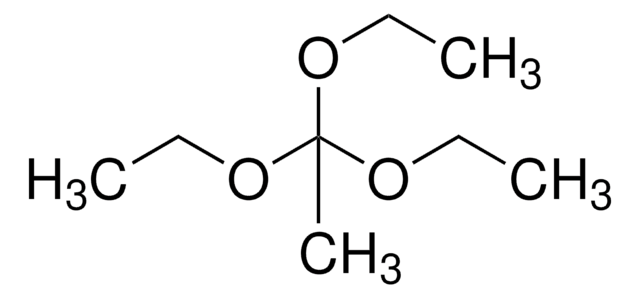
Trimethyl orthoacetate
99%
Synonym(s):
1,1,1-Trimethoxyethane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3C(OCH3)3
CAS Number:
Molecular Weight:
120.15
Beilstein:
1098338
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.388 (lit.)
bp
107-109 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
COC(C)(OC)OC
InChI
1S/C5H12O3/c1-5(6-2,7-3)8-4/h1-4H3
InChI key
HDPNBNXLBDFELL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Kinetics and mechanism of gas-phase elimination of trimethyl orthoacetate has been examined over the temperature range of 310-369°C and pressure range of 50-130Torr.
Trimethyl orthoacetate is used to synthesize acrylonitrile intermediates via reaction with alcohols followed by Knoevenagel condensation.
Trimethyl orthoacetate is used to synthesize acrylonitrile intermediates via reaction with alcohols followed by Knoevenagel condensation.
Application
Trimethyl orthoacetate has been used in the preparation of 2-amino-9-(3-acyloxymethyl-4-alkoxycarbonyloxybut-1-yl)purines and 2-amino-9-(3-alkoxycarbonyl-oxymethyl-4-alkoxycarbonyloxybut -1-yl)purines.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Sens. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
55.4 °F - closed cup
Flash Point(C)
13 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Takahide Murakami et al.
Acta biomaterialia, 84, 257-267 (2018-12-12)
Postoperative adhesion is a relevant clinical problem that causes a variety of clinical complications after abdominal surgery. The objective of this study is to develop a liquid-type antiadhesion agent and evaluate its efficacy in preventing tissue adhesion in a rat
Transesterification of trimethyl orthoacetate: an efficient protocol for the synthesis of 4-alkoxy-2-aminothiophene-3-carbonitriles
Rao KV, et al.
Tetrahedron Letters, 54, 1274-1278 (2013)
D K Kim et al.
Bioorganic & medicinal chemistry, 7(8), 1715-1725 (1999-09-11)
A series of 2-amino-9-(3-acyloxymethyl-4-alkoxycarbonyloxybut-1-yl)purin es (1-8) and 2-amino-9-(3-alkoxycarbonyl-oxymethyl-4-alkoxycarbonyloxybut -1-yl)purines (9-12) were synthesized as potential prodrugs of penciclovir. Treatment of 6-deoxypenciclovir with trimethyl orthoacetate or triethyl orthopropionate (1.2 equiv) in DMF in the presence of p-TsOH.H2O (0.1 equiv) followed by quenching
Edgar Márquez et al.
The journal of physical chemistry. A, 112(47), 12140-12142 (2008-11-01)
The gas-phase elimination kinetics of the title compounds have been examined over the temperature range of 310-369 degrees C and pressure range of 50-130 Torr. The reactions, in seasoned vessels, are homogeneous, unimolecular, and follow a first-order rate law. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service