141712
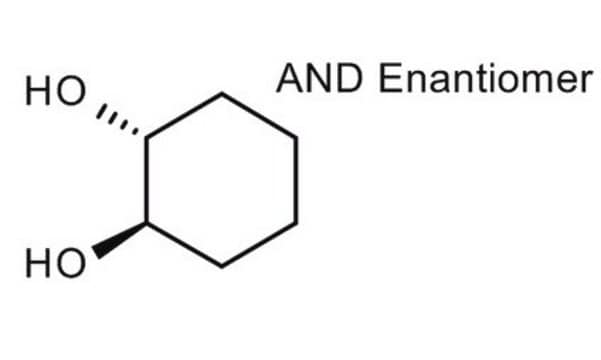
trans-1,2-Cyclohexanediol
98%
Synonym(s):
1,2-trans -Cyclohexanediol, 1,2-trans -Dihydroxycyclohexane, trans -2-Hydroxycyclohexanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H10(OH)2
CAS Number:
Molecular Weight:
116.16
Beilstein:
3193810
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
98%
form
solid
mp
101-104 °C (lit.)
SMILES string
O[C@@H]1CCCC[C@H]1O
InChI
1S/C6H12O2/c7-5-3-1-2-4-6(5)8/h5-8H,1-4H2/t5-,6-/m1/s1
InChI key
PFURGBBHAOXLIO-PHDIDXHHSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Lisa D Cervia et al.
Molecular therapy. Nucleic acids, 11, 263-271 (2018-06-03)
The nuclear envelope is a physiological barrier to electrogene transfer. To understand different mechanisms of the nuclear entry for electrotransfected plasmid DNA (pDNA), the current study investigated how manipulation of the mechanisms could affect electrotransfection efficiency (eTE), transgene expression level
R J Swift et al.
Applied microbiology and biotechnology, 55(6), 721-726 (2001-08-30)
Benzene dioxygenase (BDO; EC 1.14.12.3) from Pseudomonas putida ML2 dihydroxylates benzene to produce cis-1,2-dihydroxy-cyclohexa-3,5-diene. As well as oxidising benzene and toluene, cell-free extracts of Escherichia coli JM109 expressing recombinant BDO oxidised cyclohexene, 1-methylcyclohexene and 3-methylcyclohexene. In an attempt to construct
Andreas Hartung et al.
The Journal of organic chemistry, 72(26), 10235-10238 (2007-11-16)
Comparison is made between the preparation of trans-1,2-cyclohexanediol in standard glassware (conventional batch production) and in a microreactor (continuous flow production). The reaction sequence involved two exothermic steps where the standard procedure demands slow reagent addition and careful temperature control.
T Kiguchi et al.
Chemical & pharmaceutical bulletin, 48(10), 1536-1540 (2000-10-25)
Asymmetric spirocyclization based on intramolecular conjugate addition using a combination of a Lewis acid and an optically active cyclohexane-1,2-diol has been studied in connection with 1) the effect of substituents on the cyclohexane-1,2-diol and 2) the effect of substituents on
Ivan Liashkovich et al.
Journal of controlled release : official journal of the Controlled Release Society, 160(3), 601-608 (2012-03-06)
The efficiency of gene therapy in non-dividing cells is particularly poor due to restricted nuclear delivery rates of exogenously applied macromolecules across the nuclear pore complexes (NPCs). Therefore, improved intranuclear delivery of transgenes requires an ability to modulate the barrier
Chromatograms
suitable for GCOur team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service