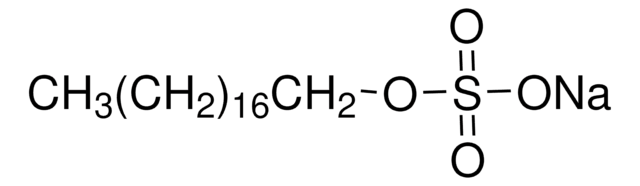106410
1-Hexadecanesulfonic acid sodium salt
98%
Synonym(s):
Sodium hexadecanesulfonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3(CH2)15SO3Na
CAS Number:
Molecular Weight:
328.49
Beilstein:
3920654
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
solubility
H2O: soluble
SMILES string
[Na+].CCCCCCCCCCCCCCCCS([O-])(=O)=O
InChI
1S/C16H34O3S.Na/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-20(17,18)19;/h2-16H2,1H3,(H,17,18,19);/q;+1/p-1
InChI key
PNGBYKXZVCIZRN-UHFFFAOYSA-M
General description
1-Hexadecanesulfonic acid sodium salt is an anionic surfactant and was determined spectrophotometrically by incorporating ion associate of anionic sulphonepthalein dye with surfactant into precipitate of chitosan.
Application
1-Hexadecanesulfonic acid sodium salt can be used as an emulsifier during the reverse iodine transfer – emulsion polymerization of styrene. It was used to study the mechanism for the crosslinking of hydroxyl-terminated poly(dimethylsiloxane).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Koichi Yamamoto et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 19(8), 1133-1138 (2003-08-30)
A spectrophotometric method for the determination of ionic surfactants with Bromophenol Blue (BPB) based on incorporation into a precipitated chitosan was studied. Cationic surfactants (CS+), such as a quaternary ammonium ion containing a long-chain alkyl group, associate with BPB2- buffered
Poly (dimethylsiloxane) coatings for controlled drug release. II. Mechanism of the crosslinking reaction in emulsion.
Gao Z, et al.
Journal of Applied Polymer Science, 91(4), 2186-2194 (2004)
[Analytical study of drugs from the phenothiazine derivative group. XVII. Sodium cetylsulphate as a titrating agent in a heterogenous medium].
J Blazek et al.
Ceskoslovenska farmacie, 28(9-10), 367-373 (1979-12-01)
R C Carroll et al.
Biochimica et biophysica acta, 777(1), 28-36 (1984-10-17)
The effects of lysoPC, four other amphiphiles containing a linear 16 carbon alkane tail and chlorpromazine on platelet cytoskeletal assembly were compared. LysoPC and nonmetabolized amphiphiles all caused time-dependent inhibition followed by potentiation of thrombin-induced aggregation, serotonin secretion and cytoskeletal
Hyeong Geun Oh et al.
Journal of colloid and interface science, 353(2), 459-466 (2010-10-19)
The RITP-emulsion polymerization of styrene in the presence of molecular iodine has been successfully performed using potassium persulfate (KPS) as an initiator and 1-hexadecanesulfonate as an emulsifier under argon atmosphere at 80°C for 7 hrs in the absence of light.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service