N-038
6β-Naltrexol solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
form
liquid
feature
Snap-N-Spike®/Snap-N-Shoot®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
forensics and toxicology
format
single component solution
storage temp.
2-8°C
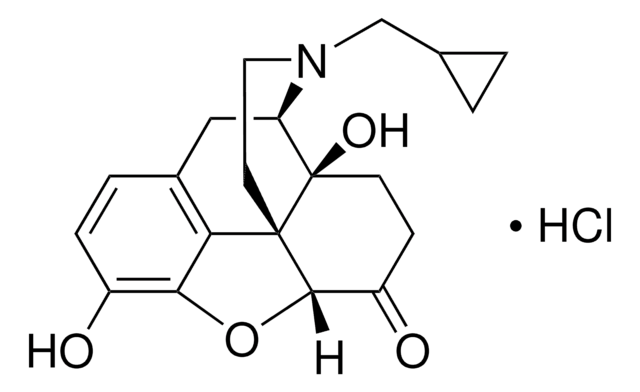
SMILES string
OC1=C(O2)C([C@]([C@@H]2[C@H](O)CC3)(CCN4CC5CC5)[C@]3(O)[C@H]4C6)=C6C=C1
InChI
1S/C20H25NO4/c22-13-4-3-12-9-15-20(24)6-5-14(23)18-19(20,16(12)17(13)25-18)7-8-21(15)10-11-1-2-11/h3-4,11,14-15,18,22-24H,1-2,5-10H2/t14-,15-,18+,19+,20-/m1/s1
InChI key
JLVNEHKORQFVQJ-PYIJOLGTSA-N
General description
Application
- Advanced analytical techniques for pharmacokinetics: The development of a rapid and sensitive gas chromatography-mass spectrometry method for nalmefene quantification in human plasma, pivotal for pharmacokinetic studies, underscores the importance of 6β-Naltrexol in therapeutic drug monitoring and addiction treatment research (Xie et al., 2002).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service