T82805
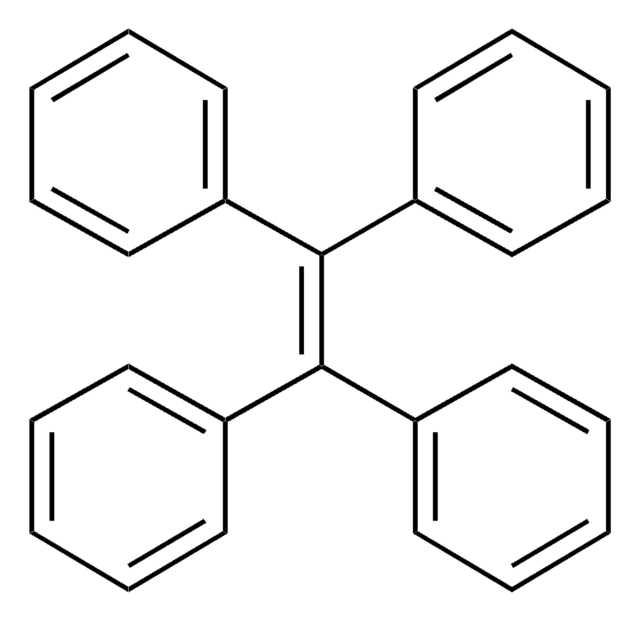
Triphenylethylene
99%
Synonym(s):
1,1,2-Triphenylethene, 1,1,2-Triphenylethylene, 1,1′,1′′-(1-Ethenyl-2-ylidene)tris[benzene], 1,2-Diphenylethenylbenzene, Benzilidenediphenylmethane, Ethene-1,1,2-triyltribenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6H5CH=C(C6H5)2
CAS Number:
Molecular Weight:
256.34
Beilstein:
1867462
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
69-71 °C (lit.)
SMILES string
c1ccc(cc1)\C=C(/c2ccccc2)c3ccccc3
InChI
1S/C20H16/c1-4-10-17(11-5-1)16-20(18-12-6-2-7-13-18)19-14-8-3-9-15-19/h1-16H
InChI key
MKYQPGPNVYRMHI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
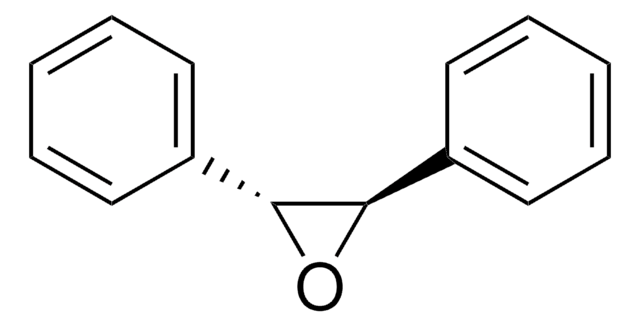
Triphenylethylene is an aromatic hydrocarbon, which can be used as a starting material to prepare 2,2,3-triphenyloxirane by asymmetric epoxidation reaction using fluorous chiral manganese complex as a catalyst. It is also used to prepare dihydro-4,5,5-triphenyl-2(3H)-furanone by reacting with acetic anhydride in the presence of MnO2 and NaOAc.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Bignon et al.
FEBS letters, 271(1-2), 54-58 (1990-10-01)
The activation of type I (gamma), II (beta) and III (alpha) protein kinase C (PKC) subspecies by phosphatidylserine (PS) and diacylglycerol (DAG) is inhibited by micromolar concentrations of triphenylacrylonitrile (TPE) antiestrogens. TPE A (with p-hydroxy and p-diethylaminoethoxy groups on the
Asymmetric epoxidation of alkenes in fluorinated media, catalyzed by second-generation fluorous chiral (Salen) manganese complexes
Cavazzini M, et al.
European Journal of Organic Chemistry, 2001(24), 4639-4649 (2001)
S J Gatley et al.
International journal of radiation applications and instrumentation. Part B, Nuclear medicine and biology, 18(7), 769-775 (1991-01-01)
A triphenylethylene compound [1,1-bis(4-hydroxyphenyl)-2-iodo-2-phenylethylene; IBHPE] has been labeled by halodestannylation with 123I at a specific radioactivity of 13,200 Ci/mmol (by in vitro receptor assay) after HPLC purification. The corresponding 80mBr-labeled compound (BrBHPE), which has a 3-fold higher affinity for the
Chellakkan S Blesson et al.
Steroids, 71(11-12), 993-1000 (2006-09-13)
The study was aimed to investigate the interaction of D,L-ormeloxifene (Orm), a triphenylethylene and its hydroxy derivative with estrogen receptor subtypes alpha and beta, its influence on ERE-driven transcriptional activation and progesterone receptor expression. In competitive binding experiments using human
Yuliya Dobrydneva et al.
Journal of cardiovascular pharmacology, 50(4), 380-390 (2007-12-01)
The anti-estrogenic drug tamoxifen, which is used therapeutically for treatment and prevention of breast cancer, can lead to the development of thrombosis. We found that tamoxifen rapidly increased intracellular free calcium [Ca2+]i in human platelets from both male and female
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service