P14858
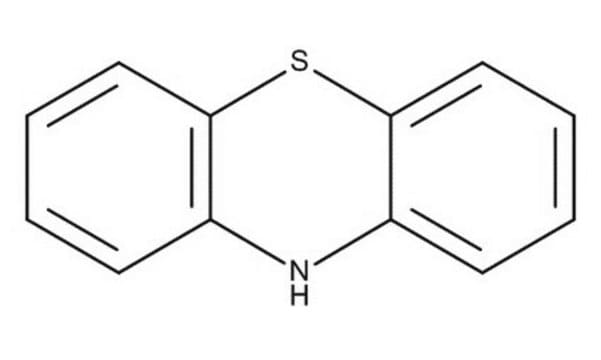
Phenoxazine
97%
Synonym(s):
5,6-Dibenzo-1,4-oxazine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H9NO
CAS Number:
Molecular Weight:
183.21
Beilstein:
143234
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
156-159 °C (lit.)
SMILES string
N1c2ccccc2Oc3ccccc13
InChI
1S/C12H9NO/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8,13H
InChI key
TZMSYXZUNZXBOL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Karina Mondragón-Vásquez et al.
Chemical communications (Cambridge, England), (44)(44), 6726-6728 (2009-11-04)
N(6)-(N'-Arylcarbamoyl)-2'-deoxyadenosine-H-phosphonates displayed molecular recognition towards cationic phenothiazinium and phenoxazinium dyes in aqueous solutions; studies have shown that binding is driven mainly by aromatic interactions and that size and shape-complementarity of the aromatic rings in host and guest provides selectivity.
Guo-Xiang Li et al.
Journal of biochemical and molecular toxicology, 23(4), 280-286 (2009-08-26)
Phenothiazine (PtzNH) and phenoxazine (PozNH) can protect human erythrocytes against hemolysis induced by 2,2'-azobis(2-amidinopropane hydrochloride) (AAPH), a peroxyl radical supplier. However, an antioxidant may be a pro-oxidant to accelerate the oxidation in the presence of radicals. The aim of this
Seelam Venkata Reddy et al.
Bioorganic & medicinal chemistry, 21(7), 1952-1963 (2013-02-19)
A number of novel imidazophenoxazine-4-sulfonamides have been designed as potential inhibitors of PDE4. All these compounds were readily prepared via an elegant multi-step method involving the initial construction of 1-nitro-10H-phenoxazine ring and then fused imidazole ring as key steps. Some
Praew Thansandote et al.
The Journal of organic chemistry, 75(10), 3495-3498 (2010-04-29)
A rapid, four-step approach to alkyl- and aryl-substituted benzomorpholines is accomplished by a Pd-catalyzed domino C-C/C-N bond coupling using a norbornene template. Extension to phenoxazines and dihydrodibenzoxazepines is presented.
Riyad Ahmed Al-Okab et al.
Journal of hazardous materials, 170(1), 292-297 (2009-05-30)
Novel, sensitive and rapid spectrophotometric methods, using phenoxazine (PNZ), 2-chlorophe-noxazine (CPN) and 2-trifluoromethylphenoxazine (TPN) as chromogenic reagents for the determination of residual chlorine are proposed. The methods are based on the reduction of chlorine by an electrophilic coupling reagent, 3-methyl-2-benzothiazoline
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
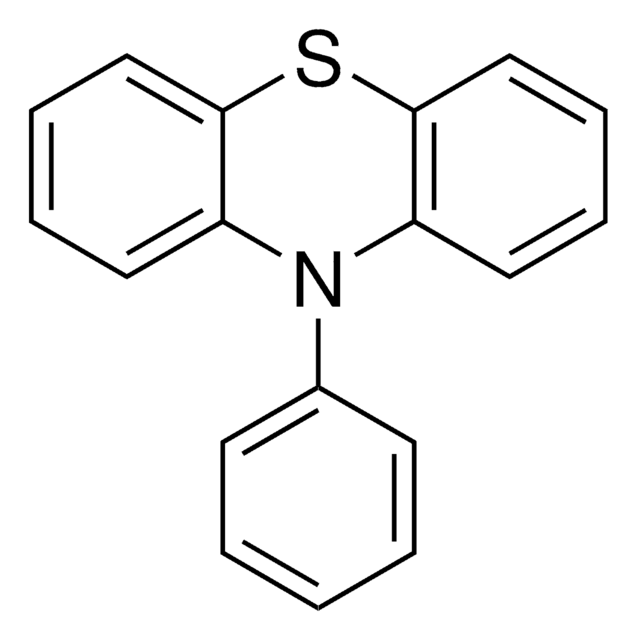
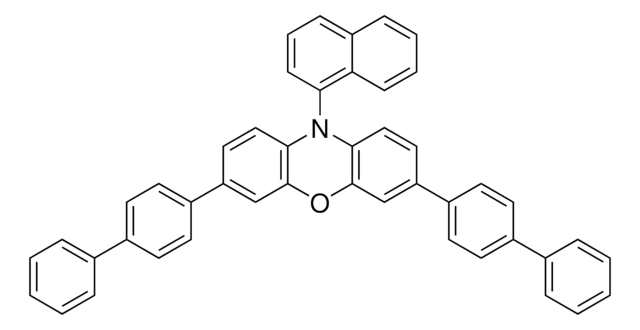
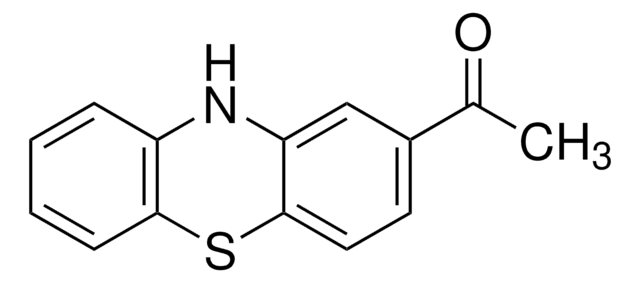




![10,11-Dihydro-5H-dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/282/468/27ed6f23-3d01-4628-8293-f0051a6f3b7c/640/27ed6f23-3d01-4628-8293-f0051a6f3b7c.png)
