G11004
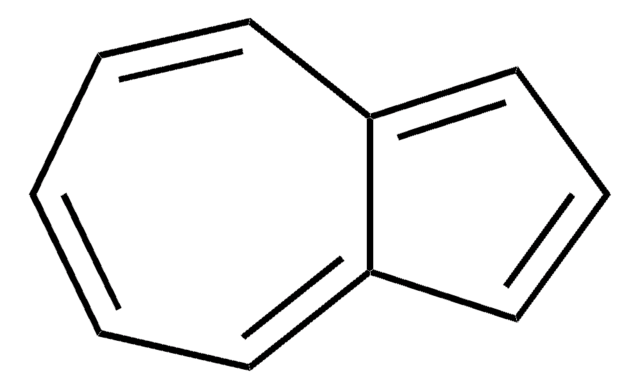
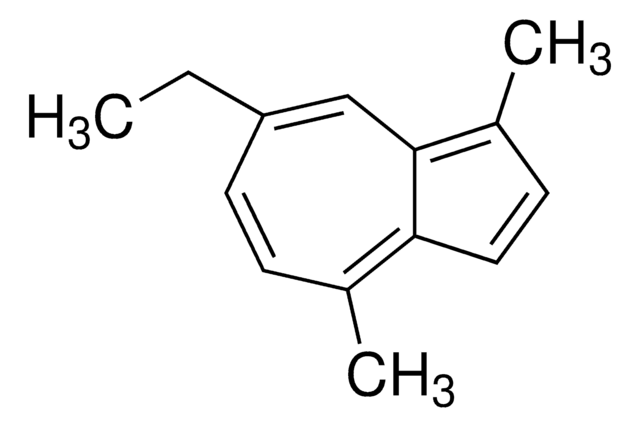
Guaiazulene
99%
Synonym(s):
1,4-Dimethyl-7-isopropylazulene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H18
CAS Number:
Molecular Weight:
198.30
Beilstein:
1365001
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
153 °C/7 mmHg (lit.)
mp
27-29 °C (lit.)
density
0.976 g/mL at 25 °C (lit.)
SMILES string
CC(C)c1ccc(C)c2ccc(C)c2c1
InChI
1S/C15H18/c1-10(2)13-7-5-11(3)14-8-6-12(4)15(14)9-13/h5-10H,1-4H3
InChI key
FWKQNCXZGNBPFD-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Guaiazulene can be used as a starting material for the synthesis of:
- Azulene-based dye molecules such as 3-(7-isopropyl-1,4-dimethylazulen-3-yl)-2-cyanoacrylic acid and 5-(7-isopropyl-1,4-dimethylazulen-3-yl)-2-cyanopenta-2,4-dienoic acid.
- Stilbazulenyl nitrone, a second-generation azulenyl nitrone which can be used as a chain-breaking antioxidant.
- Bis-azulenyl based near-infrared fluorescence quencher.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Determination of the ophthalmic drug guaiazulene by high-performance liquid chromatography.
E Vidal-Ollivier et al.
Journal of chromatography, 463(1), 227-228 (1989-01-20)
Graziano Guella et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 9(23), 5770-5777 (2003-12-16)
It is shown here that calenzanane sesquiterpenes (1 and 6) can be isolated from organic extracts from the red seaweed Laurencia microcladia Kützing from the Bay of Calenzana, Elba Island, provided contact with acidic media is minimized. Such contact induces
Jessica Fiori et al.
Toxicology in vitro : an international journal published in association with BIBRA, 25(1), 64-72 (2010-09-22)
Guaiazulene (GA) is widely used as a natural ingredient in many health care products and solutions. Although it has been reported to have interesting biological effects, GA and azulene derivatives have been proven to be cytotoxic against normal human cells
E Dovolou et al.
Reproduction in domestic animals = Zuchthygiene, 46(5), 862-869 (2011-02-18)
Reactive oxygen species (ROS) are between the major contributors for the reduced rate of in vitro bovine embryo production. It is believed that they can cause abnormal meiosis of oocytes, developmental arrest or cell death of embryos. Reports on the
Lei Wang et al.
Mutation research, 530(1-2), 19-26 (2003-10-18)
The photomutagenicity of the popular skin conditioning agents azulene and guaiazulene were tested in Salmonella typhimurium TA98, TA100 and TA102. Following irradiation with UVA and/or visible light, both azulene and guaiazulene exhibited mutagenicity 4-5-fold higher than the spontaneous background mutation.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service