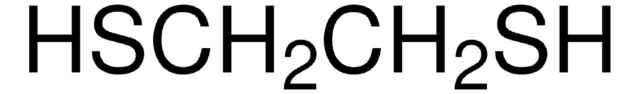467979
1,3-Dithiolane
97%
Synonym(s):
1,3-Dithiacyclopentane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H6S2
CAS Number:
Molecular Weight:
106.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.599 (lit.)
bp
183 °C (lit.)
density
1.235 g/mL at 25 °C (lit.)
functional group
thioether
SMILES string
C1CSCS1
InChI
1S/C3H6S2/c1-2-5-3-4-1/h1-3H2
InChI key
IMLSAISZLJGWPP-UHFFFAOYSA-N
General description
1,3-Dithiolane ,a sulfur containing heterocyclic building block, is a cyclic thioether. Fragmentation modes of 1,3-oxathiolane under electron-impact have been investigated.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Differentiation between carbonyls and acetals in 1, 3-dithiane and 1, 3-dithiolane synthesis catalyzed by organotin triflates.
Sato T, et al.
The Journal of Organic Chemistry, 58(18), 4971-4978 (1993)
Elemental fluorine. Part 5. Reactions of 1, 3-dithiolanes and thioglycosides with fluorine-iodine mixtures.
Chambers RD, et al.
Journal of the Chemical Society. Perkin Transactions 1, 16, 1941-1944 (1996)
Ionization and dissociation of cyclic ethers and thioethers by electron-impact. A comparison between 1, 3-dioxolane, 1, 3-dithiolane and 1, 3-oxathiolane.
Conde-Caprace G and Collin JE.
Org. Mass Spectrom., 6(4), 415-423 (1972)
I M Hussaini et al.
Acta pharmacologica Sinica, 21(10), 897-904 (2001-08-15)
To investigate the effect of a group of novel synthetic dithiolane analogs of lignans and a well characterized platelet-activating factor (PAF) receptor antagonist, L659,989 on PAF-receptor binding, IFN-gamma- and lipopolysaccharide (LPS)-induced NO production, and steady-state inducible nitric-oxide synthase (iNOS) mRNA
Alexandra Papanikos et al.
Journal of combinatorial chemistry, 6(2), 181-195 (2004-03-09)
A synthetic strategy for the formation of resin-bound internal alpha-keto amide peptides suitable for protease inhibitor screening on solid support is presented. This general approach is based on the incorporation of alpha-keto amide building blocks during solid-phase peptide synthesis (SPPS).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service