412287
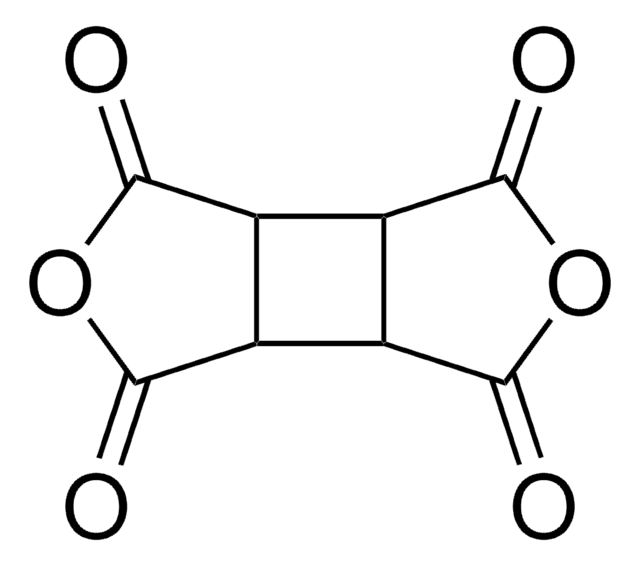
Pyromellitic dianhydride
97%
Synonym(s):
Benzene-1,2,4,5-tetracarboxylic dianhydride, PMDA
About This Item
Recommended Products
Quality Level
Assay
97%
form
powder
bp
397-400 °C (lit.)
mp
283-286 °C (lit.)
SMILES string
O=C1OC(=O)c2cc3C(=O)OC(=O)c3cc12
InChI
1S/C10H2O6/c11-7-3-1-4-6(10(14)16-8(4)12)2-5(3)9(13)15-7/h1-2H
InChI key
ANSXAPJVJOKRDJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Pyromellitic dianhydride (PMDA) is an acidic anhydride that can be used as a repair agent and as a chain extender in the formation of polyethylene terephthalate (PET) based chain extensions. It is mainly used in the production of thermoplastics and other coating applications.
Application
- A monomer to synthesize aromatic polyimides with excellent thermo-mechanical and chemical properties. These polymers find the applications in automotive and electronic industries.
- A capping agent in the development of siloxane-based hybrid materials for potential usage in organic electronics.
- A monomer in the preparation and modification of thin film composite membranes, which are used in water purification, gas separation, and biomedical devices.
- A monomer in the synthesis of pyromellitic diimide-based copolymers as stable cathode active materials for lithium and sodium-ion batteries.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
716.0 °F - closed cup
Flash Point(C)
380 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![Bicyclo[2.2.2]oct-7-ene-2,3,5,6-tetracarboxylic dianhydride 99%](/deepweb/assets/sigmaaldrich/product/structures/418/038/9edd3533-0f32-442c-8a1f-4e154e65c3b5/640/9edd3533-0f32-442c-8a1f-4e154e65c3b5.png)