316393
Sodium triacetoxyborohydride
97%
Synonym(s):
STAB
About This Item
Recommended Products
Quality Level
Assay
97%
reaction suitability
reagent type: reductant
greener alternative product score
old score: 5
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Waste Prevention
Design for Energy Efficiency
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
116-120 °C (dec.) (lit.)
greener alternative category
SMILES string
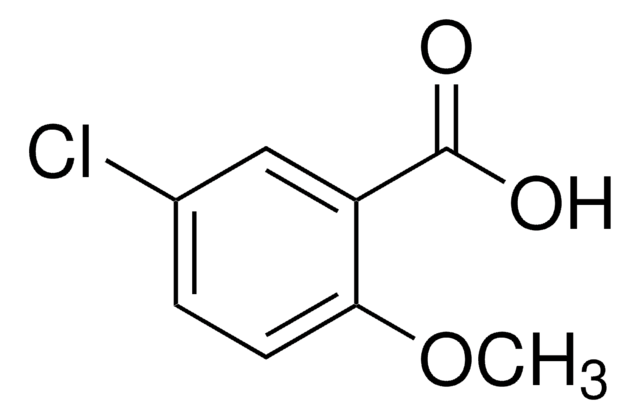
[Na+].CC(=O)O[BH-](OC(C)=O)OC(C)=O
InChI
1S/C6H10BO6.Na/c1-4(8)11-7(12-5(2)9)13-6(3)10;/h7H,1-3H3;/q-1;+1
InChI key
HHYFEYBWNZJVFQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- In the reductive amination of ketones and aldehydes.
- To prepare N-benzyl-γ-valerolactam by reacting with methyl 4-oxopentanoate and benzylamine via reductive amination/lactamization.
- To reduce imines and enamines to corresponding amines.
- To reduce quinolines and isoquinolines to corresponding tetrahydro derivatives.
- In the hydroboration of alkenes.
- To synthesize nitroxide biradicals for creating high relaxivity terminal groups linkage to dendrimers.
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Sol. 1 - Repr. 1B - Water-react 1
Supplementary Hazards
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Sodium triacetoxyborohydride
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)