247359
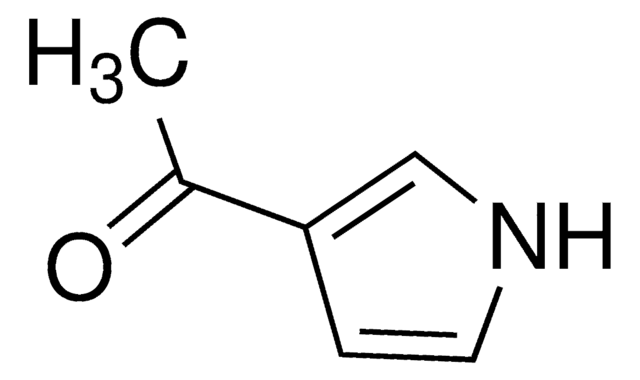
2-Acetylpyrrole
ReagentPlus®, 99%
Synonym(s):
Methyl 2-pyrrolyl ketone
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H7NO
CAS Number:
Molecular Weight:
109.13
Beilstein:
1882
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
product line
ReagentPlus®
Assay
99%
bp
220 °C (lit.)
mp
88-93 °C (lit.)
SMILES string
CC(=O)c1ccc[nH]1
InChI
1S/C6H7NO/c1-5(8)6-3-2-4-7-6/h2-4,7H,1H3
InChI key
IGJQUJNPMOYEJY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Acetylpyrrole undergoes alkylation reaction with alkyl iodide in benzene/solid KOH system in the presence of 18-crown-6 to yield the corresponding 1-alkyl derivative.
Application
2-Acetylpyrrole has been used in the synthesis of 2-acetyl-1-pyrroline.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Alkylation of 2-Acetylpyrrole and 1-alkyl-2-Acetylpyrroles Under Solid/Liquid Phase-Transfer Conditions.
Goldberg Y, et al.
Synthetic Communications, 21(4), 557-562 (1991)
Cooked rice aroma and 2-acetyl-1-pyrroline.
Buttery RG, et al.
Journal of Agricultural and Food Chemistry, 31(4), 823-826 (1983)
Wen-Yong Liu et al.
Journal of Asian natural products research, 5(3), 159-163 (2003-08-23)
Three pyrrole alkaloids were isolated from Bolbostemma paniculatum. Their structures were elucidated as 4-(2-formyl-5-methoxymethylpyrrol-1-yl)butyric acid methyl ester (1), 2-(2-formyl-5-methoxymethylpyrrol-1-yl)-3-phenylpropionic acid methyl ester (2) and alpha-methyl pyrrole ketone (3) by spectroscopic techniques. Among them, 1 and 2 are new compounds.
Jin Ho Lee et al.
The Journal of organic chemistry, 78(3), 1283-1288 (2013-01-08)
A new synthetic route to indolizines with various substituents on the pyridine moiety was developed by utilizing a facile cycloaromatization of 2-acetylpyrrole derivatives. Without isolation, the resulting intermediates were allowed to react with various electrophiles to afford a range of
G C Yen et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 24(12), 1303-1308 (1986-12-01)
Three 2-substituted pyrroles (2-acetylpyrrole, pyrrole-2-carboxaldehyde and pyrrole-2-carboxylic acid), which are products of the Maillard browning reaction, were reacted with nitrite in buffer solution (pH 3) at 50 degrees C for 24 hr. The reaction mixtures were extracted with methylene chloride
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service