120251
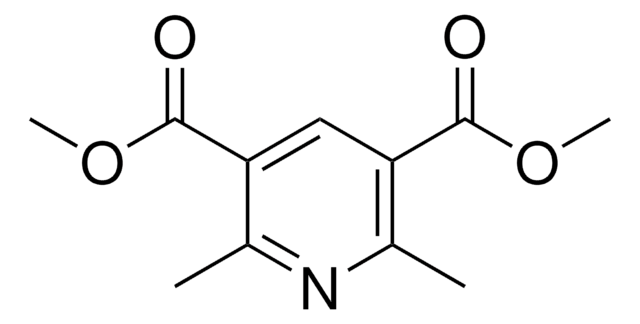
Diethyl 2,6-dimethylpyridine-3,5-dicarboxylate
99%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C13H17NO4
CAS Number:
Molecular Weight:
251.28
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
bp
208 °C/40 mmHg (lit.)
mp
72-74 °C (lit.)
functional group
ester
SMILES string
CCOC(=O)c1cc(C(=O)OCC)c(C)nc1C
InChI
1S/C13H17NO4/c1-5-17-12(15)10-7-11(13(16)18-6-2)9(4)14-8(10)3/h7H,5-6H2,1-4H3
InChI key
DIIWSYPKAJVXBV-UHFFFAOYSA-N
General description
Diethyl 2,6-dimethylpyridine-3,5-dicarboxylate is formed during the oxidation of 4-substituted Hantsch dihydropyridines in presence of methanesulfonic acid, sodium nitrite and wet SiO2 as oxidizing agents.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Aromatization of 1, 4-dihydropyridines in the presence of methanesulfonic acid/NaNO2/wet SiO2 under both heterogeneous and solvent free conditions.
Niknam K, et al.
Journal of Heterocyclic Chemistry, 43(1), 199-199 (2006)
Photooxidation of 1, 4-dihydropyridines.
Mitsunobu O, et al.
Bulletin of the Chemical Society of Japan, 45, 1453-1457 (1972)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service