All Photos(2)
About This Item
Linear Formula:
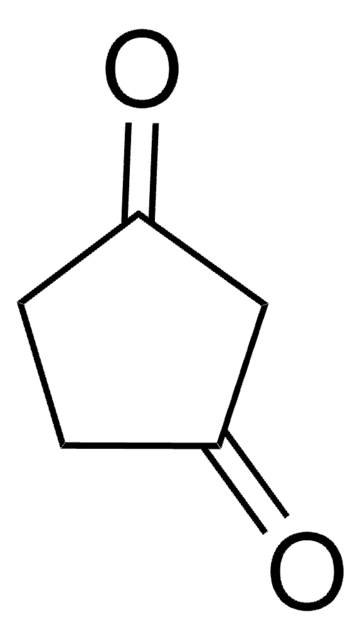
CH3C5H5(=O)2
CAS Number:
Molecular Weight:
112.13
Beilstein:
1237255
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
212-215 °C (lit.)
functional group
ketone
SMILES string
CC1C(=O)CCC1=O
InChI
1S/C6H8O2/c1-4-5(7)2-3-6(4)8/h4H,2-3H2,1H3
InChI key
HXZILEQYFQYQCE-UHFFFAOYSA-N
Gene Information
human ... ACHE(43) , BCHE(590) , CES1(1066)
Looking for similar products? Visit Product Comparison Guide
Application
2-Methyl-1,3-cyclopentanedione hs been used to explore deoxycholic acid (DCA) induced changes in cell signaling. DCA is a secondary bile acid implicated in numerous pathological conditions. It has also been used in the synthesis of (-)-curcumanolide A and (-)-curcumalactone by aldol-lactonization.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Carolyn A Leverett et al.
Journal of the American Chemical Society, 134(32), 13348-13356 (2012-08-03)
Dyotropic rearrangements of fused, tricyclic β-lactones are described that proceed via unprecedented stereospecific, 1,2-acyl migrations delivering bridged, spiro-γ-butyrolactones. A unique example of this dyotropic process involves a fused bis-lactone possessing both β- and δ-lactone moieties which enabled rapid access to
Bryson W Katona et al.
The Journal of organic chemistry, 72(24), 9298-9307 (2007-10-26)
Deoxycholic acid (DCA) is an endogenous secondary bile acid implicated in numerous pathological conditions including colon cancer formation and progression and cholestatic liver disease. DCA involvement in these disease processes results partly from its ability to modulate signaling cascades within
Randy M Wadkins et al.
Journal of medicinal chemistry, 48(8), 2906-2915 (2005-04-15)
Carboxylesterases (CE) are ubiquitous enzymes responsible for the metabolism of xenobiotics. Because the structural and amino acid homology among esterases of different classes, the identification of selective inhibitors of these proteins has proved problematic. Using Telik's target-related affinity profiling (TRAP)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service