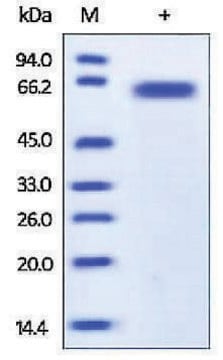
F2677
Furin human
≥2,000 unit/mL, buffered aqueous solution, recombinant, expressed in baculovirus infected Sf9 cells
Synonym(s):
Dibasic-processing enzyme, Furin convertase, PACE, Paired basic amino acid residue-cleaving enzyme
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Recommended Products
recombinant
expressed in baculovirus infected Sf9 cells
Quality Level
form
buffered aqueous solution
mol wt
57 kDa
concentration
≥2,000 unit/mL
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... FURIN(5045)
Application
Furin is capable of cleaving precursors of a wide variety of proteins, including growth factors, serum proteins, including proteases of the blood-clotting and complement systems, matrix metalloproteinases, receptors, viral-envelope glycoproteins, and bacterial exotoxins, typically at sites marked by the consensus sequence Arg-Xaa-(Lys/Arg)-Arg.
Biochem/physiol Actions
Furin is a dibasic endoprotease that is localized in the Golgi apparatus. It has a molecular mass of 52.7 kDa. It is responsible for the proteolytic maturation of many precursor proteins in the secretory and endocytic pathways of mammalian cells. Furin cleaves precursor proteins at their paired basic amino acid processing sites. Some substrates of furin include von Willebrand factor, transforming growth factor beta 1 precursor, pro-beta-secretase and proparathyroid hormone.
Furin is a dibasic endoprotease that is localized in the Golgi apparatus. It is responsible for the proteolytic maturation of many precursor proteins in the secretory and endocytic pathways of mammalian cells.
Unit Definition
One unit is defined as the amount of enzyme required to cleave 25 μg of a MBP-FN-paramyosin-ΔSal substrate to 95% completion in 6 hours at 25°C in a total reaction volume of 25 μl.
Physical form
Solution in 10 mM MES, pH 7.0 at 25 °C, 1 mM CaCl2, 50% glycerol.
Preparation Note
Isolated from Spodoptera frugiperda (Sf9) cells infected with recombinant baculovirus carrying truncated human furin
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jae-Wan Jung et al.
Plant biotechnology journal, 20(7), 1363-1372 (2022-03-25)
We have investigated the use of transient expression to produce virus-like particles (VLPs) of severe acute respiratory syndrome coronavirus 2, the causative agent of COVID-19, in Nicotiana benthamiana. Expression of a native form of the spike (S) protein, either alone
Katherine L Hussmann et al.
The Journal of general virology, 95(Pt 9), 1991-2003 (2014-06-13)
The molecular basis for the increased resistance of astrocytes to a non-neuropathogenic strain of West Nile virus (WNV), WNV-MAD78, compared with the neuropathogenic strain WNV-NY remains unclear. Here, we demonstrated that the reduced susceptibility of astrocytes to WNV-MAD78 is due
D A Bravo et al.
The Journal of biological chemistry, 269(41), 25830-25837 (1994-10-14)
Maturation of the insulin proreceptor in a late Golgi compartment requires cleavage at an Arg-Lys-Arg-Arg processing site, suggesting involvement of furin, a transmembrane serine protease of the Kex2 family of processing enzymes. A genetically engineered secreted, soluble form of human
Identification of a second human subtilisin-like protease gene in the fes/fps region of chromosome 15
M.C. Kiefer et al.
Dna and Cell Biology, 10, 757-769 (1992)
ANGPTL4 sensitizes lipoprotein lipase to PCSK3 cleavage by catalyzing its unfolding.
Anne-Marie Lund Winther et al.
Journal of lipid research, 62, 100071-100071 (2021-03-28)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service