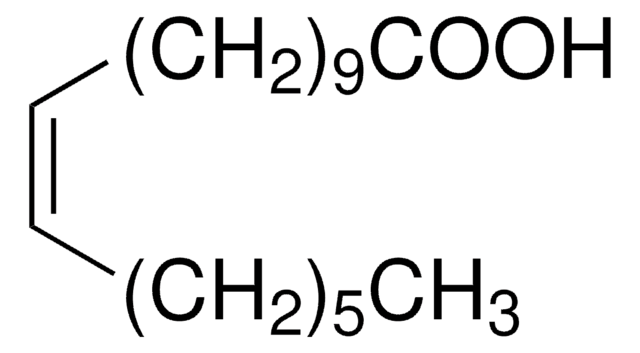216828
Iron(III) nitrate nonahydrate
ACS reagent, ≥98%
Synonym(s):
Ferric nitrate nonahydrate
About This Item
Recommended Products
grade
ACS reagent
Quality Level
Assay
≥98%
98.0-101.0% (ACS specification)
form
moist crystals
reaction suitability
reagent type: catalyst
core: iron
greener alternative product characteristics
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
impurities
≤0.005% insolubles
≤0.1% Substances not pptd. by NH4OH (as sulfates)
pH
1.5 (20 °C)
mp
47 °C (lit.)
anion traces
chloride (Cl-): ≤5 ppm
sulfate (SO42-): ≤0.01%
cation traces
Ca: ≤0.01%
K: ≤0.005%
Mg: ≤0.005%
Na: ≤0.05%
greener alternative category
, Aligned
SMILES string
O.O.O.O.O.O.O.O.O.[Fe+3].[O-][N+]([O-])=O.[O-][N+]([O-])=O.[O-][N+]([O-])=O
InChI
1S/Fe.3NO3.9H2O/c;3*2-1(3)4;;;;;;;;;/h;;;;9*1H2/q+3;3*-1;;;;;;;;;
InChI key
SZQUEWJRBJDHSM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Application
- A catalyst in the oxidation of 1o, 2o, and benzylic alcohols to their corresponding carbonyl compounds in the presence of heteropoly acids.
- A promoter in the synthesis of polycrystalline boron nitride nanotubes (BNNTs) by a ball milling-annealing method.
It can also be used to synthesize:
- Iron-doped mesoporous silica used as an efficient catalyst for the degradation of rhodamine B dye under acidic conditions.
- Iron(III)-doped titania nanoparticles by modified sol-gel method.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service