D134651
Dimethoxymethane
ReagentPlus®, 99%
Synonym(s):
Formaldehyde dimethyl acetal, Methylal
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
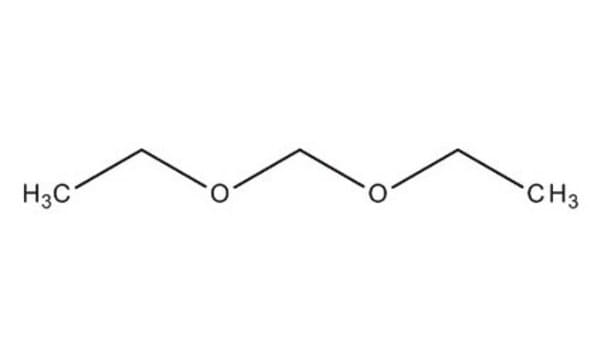
CH2(OCH3)2
CAS Number:
Molecular Weight:
76.09
Beilstein:
1697025
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
vapor density
2.6 (vs air)
Quality Level
vapor pressure
6.38 psi ( 20 °C)
product line
ReagentPlus®
Assay
99%
form
liquid
autoignition temp.
459 °F
expl. lim.
17.6 %
refractive index
n20/D 1.354 (lit.)
bp
41-42 °C (lit.)
mp
−105 °C (lit.)
density
0.86 g/mL at 25 °C (lit.)
SMILES string
COCOC
InChI
1S/C3H8O2/c1-4-3-5-2/h3H2,1-2H3
InChI key
NKDDWNXOKDWJAK-UHFFFAOYSA-N
Related Categories
General description
Dimethoxymethane (DMM, methylal) is a biodegradable dimethyl acetal. It can be synthesized by acid catalyzed condensation of formaldehyde with methanol. It is amphiphilic in nature with low viscosity, surface tension and boiling point. It is a flammable, highly volatile solvent with good dissolving power. DMM is considered as a potential alternative fuel and fuel additive due to its high oxygen content and its ability to enhance the combustion characteristics of diesel and petrol. Its thermal diffusivity has been determined by photoacoustic method. Analysis of the molecular structure of DMM by electron diffraction technique shows that it has C2 symmetry with a gauche-gauche conformation.
Application
Dimethoxymethane (Formaldehyde dimethyl acetal) may be used in the synthesis of methoxymethyl (MOM) ethers. It may also be used as an external cross-linker to form microporous polymers.
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Supplementary Hazards
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The molecular structure of dimethoxymethane.
Astrup EE.
Acta Chemica Scandinavica, 27(9), 3271-3276 (1973)
A photoacoustic study on Dimethoxymethane.
Bama GK and Ramachandran K.
AIP Conference Proceedings, 1004, 191-191 (2008)
Scandium trifluoromethanesulfonate as a recyclable catalyst for efficient methoxymethylation of alcohols.
Karimi B and Ma'mani L.
Tetrahedron Letters, 44(32), 6051-6053 (2003)
Highly efficient and selective methoxymethylation of alcohols and phenols catalyzed by high-valent tin (IV) porphyrin.
Gharaati S, et al.
Inorgorganica Chimica Acta, 363(9), 1995-2000 (2010)
Purification and dehydration of methylal by pervaporation.
Carretier E, et al.
Journal of Membrane Science , 217(1), 159-171 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service