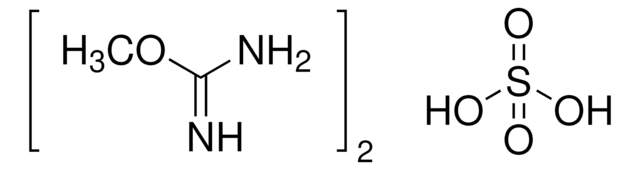M53701
o-Methylisourea bisulfate
99%
Synonym(s):
2-Methylpseudourea monosulfate (1:1), OMI®
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NC(OCH3)=NH · H2SO4
CAS Number:
Molecular Weight:
172.16
Beilstein:
3722928
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
118-120 °C (lit.)
SMILES string
COC(N)=N.OS(O)(=O)=O
InChI
1S/C2H6N2O.H2O4S/c1-5-2(3)4;1-5(2,3)4/h1H3,(H3,3,4);(H2,1,2,3,4)
InChI key
MDFRYRPNRLLJHT-UHFFFAOYSA-N
Gene Information
human ... OPRD1(4985) , OPRK1(4986) , OPRM1(4988)
rat ... Oprd1(24613) , Oprm1(25601)
Legal Information
OMI is a registered trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dmitry S Loginov et al.
Parasites & vectors, 12(1), 212-212 (2019-05-08)
The availability of tick in vitro cell culture systems has facilitated many aspects of tick research, including proteomics. However, certain cell lines have shown a tissue-specific response to infection. Thus, a more thorough characterization of tick cell lines is necessary.
Cheng Guo et al.
European journal of mass spectrometry (Chichester, England), 24(5), 384-396 (2018-07-26)
Modified peptides fragmented by collision-induced dissociation can offer additional sequence information, which is beneficial for the de novo sequencing of peptides. Here, the model peptide VQGESNDLK was carbamylated. The optimal conditions were as follows: temperature of 90℃, pH of 7
H Wojciechowska et al.
Acta biochimica Polonica, 29(3-4), 197-204 (1982-01-01)
Selectivity of amidination using ornithine as model amino acid was investigated in detail. The results obtained were taken advantage of for studying the reactions with other polyfunctional amino acids: beta-tyrosine, isoserine, 2,3-diaminopropionic acid, 2,6-diamino-7-hydroxyazelaic acid and with the pentapeptide amide
K M Fazili et al.
Biochemistry and molecular biology international, 31(5), 807-816 (1993-12-01)
Using acetic anhydride, potassium cyanate and O-methyl isourea six chemically modified derivatives of bovine serum albumin with chemical modification on lysine side chains have been prepared. All the modified preparations were found to be homogeneous with respect to size and
Complex formation of guanidinated bovine trypsin inhibitor (Kunitz) with trypsin, chymotrypsin and trypsinogen as studied by the spin-label technique.
H R Wenzel et al.
FEBS letters, 140(1), 53-57 (1982-04-05)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service