All Photos(1)
About This Item
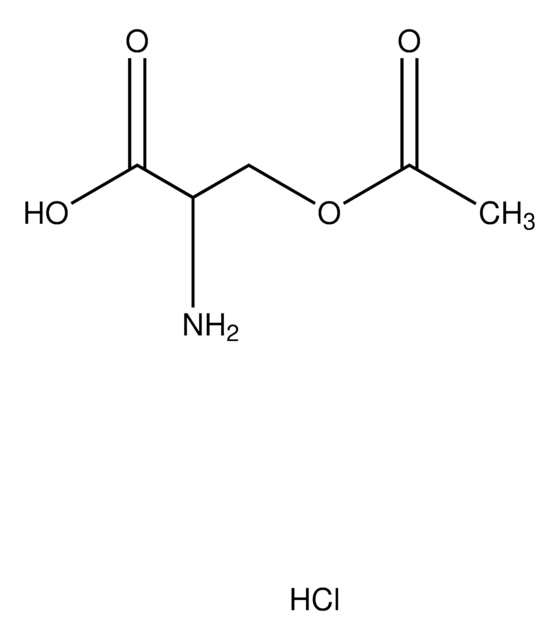
Empirical Formula (Hill Notation):
C5H9NO4
Molecular Weight:
147.13
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
description
AldrichCPR
form
solid
storage temp.
2-8°C
SMILES string
N[C@H](C(O)=O)COC(C)=O
InChI
1S/C5H9NO4/c1-3(7)10-2-4(6)5(8)9/h4H,2,6H2,1H3,(H,8,9)/t4-/m0/s1
InChI key
VZXPDPZARILFQX-BYPYZUCNSA-N
Other Notes
Please note that Sigma-Aldrich provides this product to early discovery researchers as part of a collection of unique chemicals. Sigma-Aldrich does not collect analytical data for this product. Buyer assumes responsibility to confirm product identity and/or purity. All sales are final.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY, (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY, WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
NOTWITHSTANDING ANY CONTRARY PROVISION CONTAINED IN SIGMA-ALDRICH′S STANDARD TERMS AND CONDITIONS OF SALE OR AN AGREEMENT BETWEEN SIGMA-ALDRICH AND BUYER, SIGMA-ALDRICH SELLS THIS PRODUCT "AS-IS" AND MAKES NO REPRESENTATION OR WARRANTY WHATSOEVER WITH RESPECT TO THIS PRODUCT, INCLUDING ANY (A) WARRANTY OF MERCHANTABILITY, (B) WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE, OR (C) WARRANTY AGAINST INFRINGEMENT OF INTELLECTUAL PROPERTY RIGHTS OF A THIRD PARTY, WHETHER ARISING BY LAW, COURSE OF DEALING, COURSE OF PERFORMANCE, USAGE OF TRADE OR OTHERWISE.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Robert G Mothersole et al.
Biochemistry, 59(44), 4250-4261 (2020-10-29)
Lanthionine synthase from the oral bacterium Fusobacterium nucleatum is a fold type II pyridoxal-5'-phosphate (PLP)-dependent enzyme that catalyzes the β-replacement of l-cysteine by a second molecule of l-cysteine to form H2S and l-lanthionine. The meso-isomer of the latter product is
Christof Dietzen et al.
Plant physiology, 184(4), 2120-2136 (2020-10-17)
Sulfur, an indispensable constituent of many cellular components, is a growth-limiting macronutrient for plants. Thus, to successfully adapt to changing sulfur availability and environmental stress, a sulfur-deficiency response helps plants to cope with the limited supply. On the transcriptional level
Liang Wei et al.
Applied microbiology and biotechnology, 103(3), 1325-1338 (2018-12-20)
L-cysteine, a valuable sulfur-containing amino acid, has been widely used in food, agriculture, and pharmaceutical industries. Due to the toxicity and complex regulation of L-cysteine, no efficient cell factory has yet been achieved for L-cysteine industrial production. In this study
Shrijita Banerjee et al.
BMC biochemistry, 12, 31-31 (2011-06-03)
The importance of understanding the detailed mechanism of cysteine biosynthesis in bacteria is underscored by the fact that cysteine is the only sulfur donor for all cellular components containing reduced sulfur. O-acetylserine sulfhydrylase (OASS) catalyzes this crucial last step in
Isha Nagpal et al.
PloS one, 7(2), e30305-e30305 (2012-02-23)
The explosive epidemicity of amoebiasis caused by the facultative gastrointestinal protozoan parasite Entamoeba histolytica is a major public health problem in developing countries. Multidrug resistance and side effects of various available antiamoebic drugs necessitate the design of novel antiamobeic agents.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service