B13055
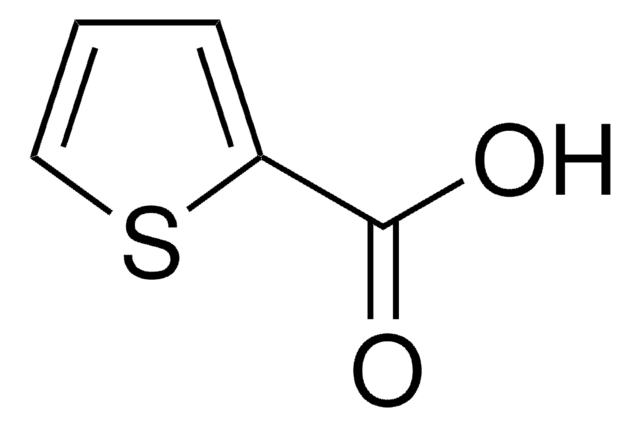
Phenylglyoxylic acid
97%
Synonym(s):
α-Oxophenylacetic acid, Benzoylformic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5COCOOH
CAS Number:
Molecular Weight:
150.13
Beilstein:
606718
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
crystals
mp
62-65 °C (lit.)
SMILES string
OC(=O)C(=O)c1ccccc1
InChI
1S/C8H6O3/c9-7(8(10)11)6-4-2-1-3-5-6/h1-5H,(H,10,11)
InChI key
FAQJJMHZNSSFSM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Phenylglyoxylic acid can be used as a precursor in the synthesis of:
- O-acyl acetanilides by decarboxylative o-acylation of acetanilides using Pd catalyst.
- Phenylhydroxycarbene by high-vacuum flash pyrolysis.
- 2-arylbenzothiazoles by reacting with o-aminothiophenol using ammonium niobium oxalate (ANO) as a catalyst.
- 3-aryl-2H-benzo[b][1,4]benzoxazin-2-ones by treating with o-aminophenol in the presence of ammonium niobium oxalate catalyst.
- 2-aryl benzothiazoles through potassium persulfate (K2S2O8)-mediated oxidative condensation of benzothiazoles.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Phenylhydroxycarbene.
Gerbig D, et al.
Journal of the American Chemical Society, 132(21), 7273-7275 (2010)
Room temperature palladium-catalyzed decarboxylative ortho-acylation of acetanilides with ?-oxocarboxylic acids.
Fang P, et al.
Journal of the American Chemical Society, 132(34), 11898-11899 (2010)
Niobium-promoted reaction of ?-phenylglyoxylic acid with ortho-functionalized anilines: synthesis of 2-arylbenzothiazoles and 3-aryl-2 H-benzo [b][1, 4] benzoxazin-2-ones
Penteado F, et al.
Green Chemistry, 18(24), 6675-6680 (2016)
Jesus M Aizpurua et al.
The Journal of organic chemistry, 74(17), 6691-6702 (2009-08-01)
Mechanistic details of the Mg(2+) ion-activated enantioselective reduction of methyl benzoylformate have been investigated at a B3LYP/6-31G* theory level, using peptide NADH models 1 rigidified with a beta-lactam ring. Computation of the reaction pathway revealed important structural differences between the
Zhi-de Zhou et al.
International journal of biological macromolecules, 47(1), 21-26 (2010-04-20)
Saccharomyces cerevisiae alcohol dehydrogenase (SCAD) was effectively immobilized on hybrid alginate-chitosan beads which are hardened with glutaraldehyde. Immobilization conditions and characterization of the immobilized enzyme were investigated. Orthogonal test design and intuitive analysis method were employed to evaluate the effects
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service