711721
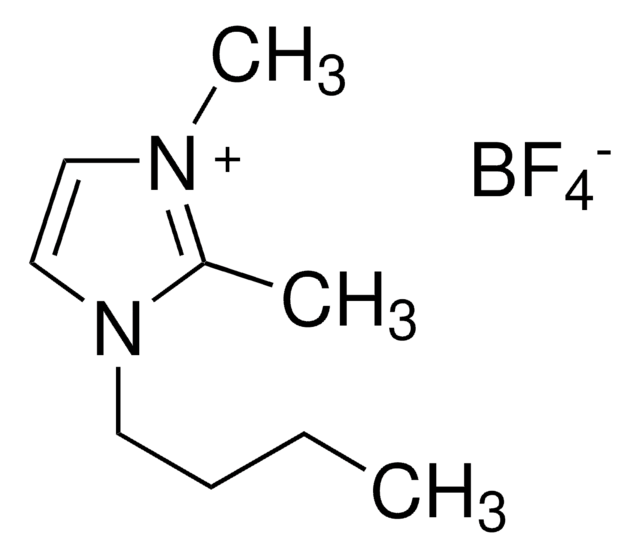
1-Ethyl-3-methylimidazolium tetrafluoroborate
≥98% (HPLC)
Synonym(s):
EMIMBF4
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H11BF4N2
CAS Number:
Molecular Weight:
197.97
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98% (HPLC)
form
liquid
impurities
≤0.5% water
refractive index
n20/D 1.413 (lit.)
bp
>350 °C (lit.)
mp
15 °C (lit.)
density
1.294 g/mL at 25 °C (lit.)
SMILES string
F[B-](F)(F)F.CCn1cc[n+](C)c1
InChI
1S/C6H11N2.BF4/c1-3-8-5-4-7(2)6-8;2-1(3,4)5/h4-6H,3H2,1-2H3;/q+1;-1
InChI key
CUNYTRQQXKCRTJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Ethyl-3-methylimidazolium tetrafluoroborate is an air and water stable, room temperature ionic liquid (RTIL). It can be prepared by reacting 1-ethyl-3-methylimidazolium bromide with tetrafluoroboric acid.
Application
- 1-Ethyl-3-methylimidazolium tetrafluoroborate-doped high ionic conductivity gel electrolytes with reduced anodic reaction potentials for electrochromic devices: This study reports the preparation of a 1-Ethyl-3-methylimidazolium tetrafluoroborate ([Emim] BF4)-doped Poly(methyl methacrylate)(PMMA)-based gel electrolyte for electrochromic applications, showing improved ionic conductivity and reduced anodic reaction potentials (Tang et al., 2017).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Flexible Asymmetric Supercapacitor Based on Structure?Optimized Mn3O4/Reduced Graphene Oxide Nanohybrid Paper with High Energy and Power Density
Hu, Yating, et al.
Advances in Functional Materials, 25.47, 7291-7299 (2015)
Crystal structures of frozen room temperature ionic liquids, 1-ethyl-3-methylimidazolium tetrafluoroborate (EMImBF 4), hexafluoroniobate (EMImNbF 6) and hexafluorotantalate (EMImTaF 6), determined by low-temperature X-ray diffraction
Matsumoto, Kazuhiko, et al.
Solid State Sciences, 8.10, 1250-1257 (2006)
Solubility of carbon dioxide in 1-ethyl-3-methylimidazolium tetrafluoroborate
Soriano, Allan N., Bonifacio T. Doma Jr, and Meng-Hui Li
Journal of Chemical and Engineering Data, 53.11, 2550-2555 (2008)
Efficient, halide free synthesis of new, low cost ionic liquids: 1, 3-dialkylimidazolium salts containing methyl-and ethyl-sulfate anions
Holbrey, John D., et al.
Green Chemistry, 4.5, 407-413 (2002)
Density and Viscosity Measurements for Binary Mixtures of 1-Ethyl-3-methylimidazolium Tetrafluoroborate ([Emim][BF4]) with Dimethylacetamide, Dimethylformamide, and Dimethyl Sulfoxide
Fan, Xian-Heng, Yan-Ping Chen, and Chie-Shaan Su.
Journal of Chemical and Engineering Data, 61.2, 920-927 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service