All Photos(1)
About This Item
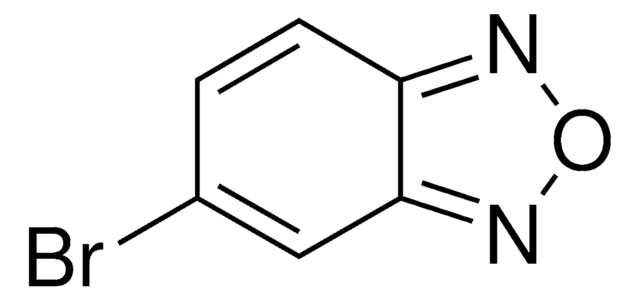
Empirical Formula (Hill Notation):
C6H4N2O
CAS Number:
Molecular Weight:
120.11
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
75-85 °C/20 mmHg (lit.)
mp
47-51 °C (lit.)
SMILES string
c1ccc2nonc2c1
InChI
1S/C6H4N2O/c1-2-4-6-5(3-1)7-9-8-6/h1-4H
InChI key
AWBOSXFRPFZLOP-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
168.8 °F - closed cup
Flash Point(C)
76 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Nathaniel T Greene et al.
Journal of the American Chemical Society, 127(15), 5695-5700 (2005-04-14)
A colorimetric sensor array composed of seven molecularly imprinted polymers was shown to accurately identify seven different aromatic amines. The response patterns were systematically classified using linear discriminant analysis with 94% classification accuracy. Analyses of the response patterns of the
Maki Onoda et al.
Luminescence : the journal of biological and chemical luminescence, 17(1), 11-14 (2002-01-30)
We studied the effects of spacer length on the fluorescence quantum yields (Phi) of photoinduced electron transfer (PET) reagents, using nitrobenzoxadiazole (NBD) derivatives that have the -NMe2 moiety and NBD-NH- fluorophore as electron donor (D) and electron acceptor (A), respectively.
Jana Rohacova et al.
ChemMedChem, 4(3), 466-472 (2009-01-29)
One of the most common mechanisms of hepatotoxicity is drug-induced cholestasis. Hence, new approaches for screening the cholestatic potential of drug candidates are desirable. In this context, we describe herein the use of synthetic 4-nitrobenzo-2-oxa-1,3-diazole (NBD) fluorescent conjugates of cholic
T Takabatake et al.
Chemical & pharmaceutical bulletin, 39(5), 1352-1354 (1991-05-01)
The superoxide (O2-.) production in Escherichia coli through the action of benzofurazans (BZs) was examined using the cytochrome c (cyt. c) reduction method. Adding BZs to E. coli cell suspensions caused the cyt. c reduction, which was completely inhibited by
T Santa et al.
Biomedical chromatography : BMC, 12(2), 73-77 (1998-05-06)
The enantiomneric separation and the detection of 2-arylpropionic acids after derivatization with the fluorescent reagents with a benzofurazan structure, (S)-(+)-4-(N,N- dimethylaminosulphonyl)-7-(3-aminopyrrolidin-1-yl)-2,1,3-ben zoxadiazole ((S)-DBD-Apy), (R)-(-)-4-nitro-7-(3-aminopyrrolidin-1-yl)-2,1,3- benzoxadiazole ((R)-NBD-Apy), 4-N,N-dimethylaminosulphonyl-7-piperazino-2,1,3-benzoxadi zole (DBD-PZ) and N-hydrazinoformylmethyl-N-methylamino-4,4- N,N-dimethylaminosulphonyl-2,1,3-benzoxadiazole (DBD-CO-Hz) by high-performance liquid chromatography (HPLC) and electrospray
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![Benzo[c][1,2,5]oxadiazole-5-boronic acid pinacol ester 97%](/deepweb/assets/sigmaaldrich/product/structures/143/941/5b091bed-2dcd-4ac5-b86d-70df392aabce/640/5b091bed-2dcd-4ac5-b86d-70df392aabce.png)