All Photos(1)
About This Item
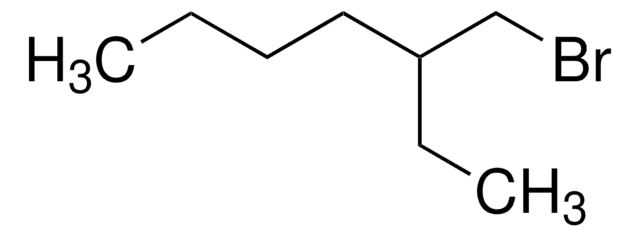
Linear Formula:
(CH3)2CH(CH2)3Br
CAS Number:
Molecular Weight:
165.07
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.446 (lit.)
bp
146 °C (lit.)
density
1.134 g/mL at 25 °C (lit.)
SMILES string
CC(C)CCCBr
InChI
1S/C6H13Br/c1-6(2)4-3-5-7/h6H,3-5H2,1-2H3
InChI key
XZKFBZOAIGFZSU-UHFFFAOYSA-N
General description
1-Bromo-4-methylpentane can be synthesized by treating 4-methyl-1-pentanol with phosphorous tribromide. The conformational analysis of its liquid-state and solid-state IR and Raman spectra and showed the presence of a mixture of PC,PH, and P′H conformers.
Application
1-Bromo-4-methylpentane may be used in the synthesis of:
- diethyl 2-(4′-methylpentyl)malonate
- 1,3-bis(4-(isopentyloxy)phenyl)urea
- 1,3-bis(4-(isopentyloxy)phenyl)thiourea
- 13-methyl-1-[(tetrahydropyran-2-yl)oxy]tetradec-8-yne
- 15-methyl-1-[(tetrahydropyran-2-yl)oxy]hexadec-10-yne
- 3-[2-isohexyloxy-3-(hydroxymethyl]-5-phenyl-2-isohexyloxybenzyl alcohol
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
73.4 °F
Flash Point(C)
23 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Takano et al.
The Journal of biological chemistry, 275(15), 11010-11016 (2001-02-07)
Early stationary phase culture supernatants of Streptomyces coelicolor A3(2) contained at least four small diffusible signaling molecules that could elicit precocious antibiotic synthesis in the producing strain. The compounds were not detected in exponentially growing cultures. One of these compounds
B Reiter et al.
Journal of chemical ecology, 29(10), 2235-2252 (2003-12-20)
Gas chromatography, coupled gas chromatography-mass spectrometry (electron impact mode and chemical ionization with methane as reactant gas), gas chromatography-infrared spectroscopy, and derivatization techniques were used to identity 53 compounds in the interdigital secretion of the red hartebeest, Alcelaphus buselaphus caama.
Néstor M Carballeira et al.
Chemistry and physics of lipids, 145(1), 37-44 (2006-11-28)
The first total syntheses for the (Z)-15-methyl-10-hexadecenoic acid and the (Z)-13-methyl-8-tetradecenoic acid were accomplished in seven steps and in 31-32% overall yields. The (trimethylsilyl)acetylene was the key reagent in both syntheses. It is proposed that the best synthetic strategy towards
Vibrational analysis of alkyl bromides: Part III. Branched-chain bromides: 1-bromo-3-methylbutane and 1-bromo-4-methylpentane.
Crowder GA and Jalilian MR.
Journal of Molecular Structure, 42, 71-76 (1977)
Maria E Amato et al.
Molecules (Basel, Switzerland), 15(3), 1442-1452 (2010-03-26)
A novel chiral macrocyclic ligand incorporating a chiral salen moiety into a framework containing two biphenyl units was synthesized. Structural properties and conformational aspects of the free ligand and an UO2 complex were studied by using NMR spectroscopy in solution
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service