36295
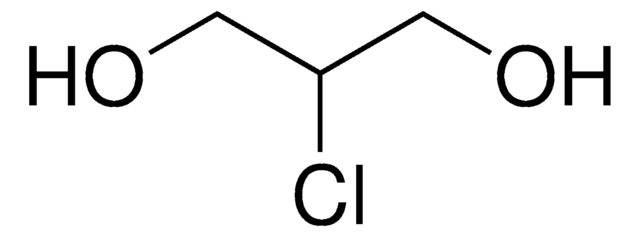
2,3-Dichloro-1-propanol
≥97.0% (GC)
Synonym(s):
Glycerol-α,β-dichlorohydrin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCH2CHClCH2OH
CAS Number:
Molecular Weight:
128.99
Beilstein:
1732060
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
density
1.360 g/mL at 20 °C (lit.)
SMILES string
OCC(Cl)CCl
InChI
1S/C3H6Cl2O/c4-1-3(5)2-6/h3,6H,1-2H2
InChI key
ZXCYIJGIGSDJQQ-UHFFFAOYSA-N
General description
2,3-Dichloro-1-propanol belongs to the group of chloropropanols. Inhibitory effects of 2,3-dichloro-1-propanol on T cell both in vivo and in vitro is reported. Improved enantioselective resolution of (R,S)-2,3-dichloro-1-propanol to (S)-2, 3-dichloro-1-propanol by whole cells of a recombinant Escherichia coli in n-heptane-aqueous biphasic system is reported.
Application
2,3-Dichloro-1-propanol may be employed as carbon and energy supplement for the growth of Pseudomonas putida strain (MC4).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Eye Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
199.4 °F
Flash Point(C)
93 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Ijaz Gul et al.
Biotechnology and applied biochemistry, 67(4), 685-692 (2020-02-18)
Epoxides are widely used chemicals, the determination of which is of paramount importance. Herein, we present an enzyme-based approach for noninstrumental detection of epoxides in standard solution and environmental samples. Halohydrin dehalogenase (HheC) as a biological recognition element and epichlorohydrin
Hassan M Badawi et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(2), 734-738 (2009-12-22)
The conformational stability and the three rotor internal rotations in 2,3-dichloro-1-propanol were investigated at DFT-B3LYP/6-311+G**, MP2/6-311+G** and MP4(SDQ) levels of theory. From the calculated potential energy surface, ten distinct minima were located all of which were predicted to have real
Comparative testicular toxicities of two isomers of dichloropropanol, 2,3-dichloro-1-propanol, and 1,3-dichloro-2-propanol, and their metabolites alpha-chlorohydrin and epichlorohydrin, and the potent testicular toxicant 1,2-dibromo-3-chloropropane.
M Omura et al.
Bulletin of environmental contamination and toxicology, 55(1), 1-7 (1995-07-01)
M Koga et al.
Journal of UOEH, 14(1), 13-22 (1992-03-01)
Urinary metabolites of dichloropropanols in rats were analyzed by gas chromatography-mass spectrometry (GC/MS). Solutions of dichloropropanols consisting of 1, 3-dichloro-2-propanol (DC2P) and 2, 3-dichloro-1-propanol (DC1P) were diluted in a saline at the concentration of 100 mg/ml, and 0.1 ml of
A H Hammond et al.
Chemico-biological interactions, 122(2), 107-115 (1999-10-21)
The effect of aldehyde dehydrogenase inhibition by cyanamide pre-treatment in vitro on dichloropropanol-dependent toxicity and glutathione depletion was investigated in 24 h rat hepatocyte cultures. Cyanamide pre-treatment had no effect on nitrophenol hydroxylase, 7-methoxy-, 7-ethoxy- or 7-benzyloxyresorufin O-dealkylase activities in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service