254509
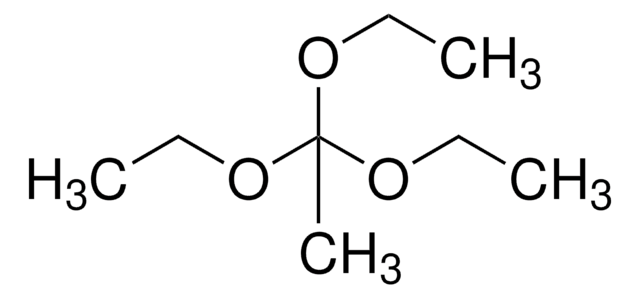
Trimethyl orthobutyrate
97%
Synonym(s):
1,1,1-Trimethoxybutane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH2CH2C(OCH3)3
CAS Number:
Molecular Weight:
148.20
Beilstein:
1737319
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.404 (lit.)
bp
145-147 °C (lit.)
density
0.926 g/mL at 25 °C (lit.)
functional group
ether
SMILES string
CCCC(OC)(OC)OC
InChI
1S/C7H16O3/c1-5-6-7(8-2,9-3)10-4/h5-6H2,1-4H3
InChI key
JAFMOTJMRSZOJE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Kinetics and mechanism of gas-phase elimination of trimethyl orthobutyrate has been examined over the temperature range of 310-369°C and pressure range of 50-130Torr.
Application
Trimethyl orthobutyrate has been used in the preparation of:
- 5-acetamido-9-O-butyroyl-3,5-dideoxy-D-glycero-2-nonulo pyranosonic acid
- 9-O-butyroyl-3,5-dideoxy-5-glycoloylamido-D-glycero-2-nonulo pyranosonic acid
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
122.0 °F - closed cup
Flash Point(C)
50 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Edgar Márquez et al.
The journal of physical chemistry. A, 112(47), 12140-12142 (2008-11-01)
The gas-phase elimination kinetics of the title compounds have been examined over the temperature range of 310-369 degrees C and pressure range of 50-130 Torr. The reactions, in seasoned vessels, are homogeneous, unimolecular, and follow a first-order rate law. The
H Ogura et al.
Carbohydrate research, 167, 77-86 (1987-09-15)
Various 9-O-acyl derivatives of N-acetyl- and N-glycoloyl-neuraminic acid, and O-(5-acetamido-3,5-dideoxy-D-glycero-alpha- and beta-D-galacto-2-nonulopyranosylonic acid)-(2----6)-O-beta-D-galactopyranosyl-(1----4)-D-glucopyranose were regioselectively synthesized by use of ortho esters. In addition, 5-acetamido-4-O-acetyl-D-glycero-D-galacto-2-nonulopyranosonic acid was prepared starting from the benzyl and methyl esters of N-acetylneuraminic acid.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service