228907
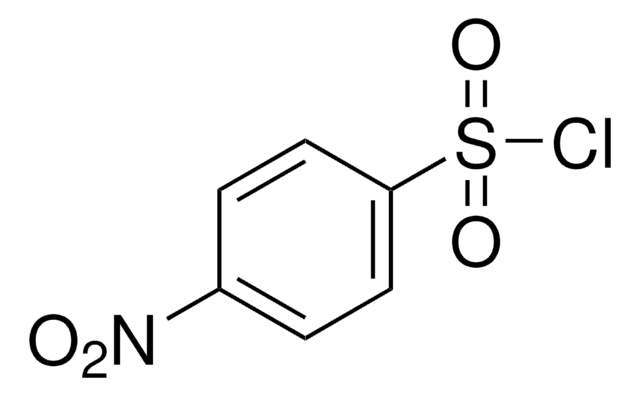
2-Nitrobenzenesulfonamide
98%
Synonym(s):
NSC 23381, NSC 629275
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4SO2NH2
CAS Number:
Molecular Weight:
202.19
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
190-192 °C (lit.)
functional group
nitro
SMILES string
NS(=O)(=O)c1ccccc1[N+]([O-])=O
InChI
1S/C6H6N2O4S/c7-13(11,12)6-4-2-1-3-5(6)8(9)10/h1-4H,(H2,7,11,12)
InChI key
GNDKYAWHEKZHPJ-UHFFFAOYSA-N
Application
Reactant involved in synthesis of:
Reactant involved in intermolecular amination of allyl alcohols
- Cyclic nitrogen compounds via intramolecular hydroamination
- Pentacyclic lycopodium alkaloid huperzine-Q
- Pyrrolidines
- Intermolecular C-H insertion reactions
Reactant involved in intermolecular amination of allyl alcohols
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Y Hidai et al.
Chemical & pharmaceutical bulletin, 48(10), 1570-1576 (2000-10-25)
Total synthesis of spider toxins HO-416b (1) and Agel-489 (2) was accomplished using the 2-nitrobenzenesulfonamide (Ns) group as both a protecting and activating group. In this strategy, the C-N bonds were constructed by alkylation of sulfonamides with alkyl halides or
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service