All Photos(2)
About This Item
Empirical Formula (Hill Notation):
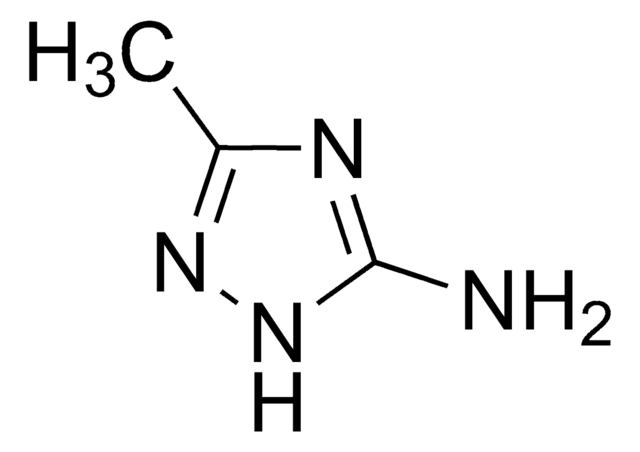
C3H6N4S
CAS Number:
Molecular Weight:
130.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
130-133 °C (lit.)
functional group
thioether
SMILES string
CSc1nc(N)n[nH]1
InChI
1S/C3H6N4S/c1-8-3-5-2(4)6-7-3/h1H3,(H3,4,5,6,7)
InChI key
XGWWZKBCQLBJNH-UHFFFAOYSA-N
General description
3-Amino-5-methylthio-1H-1,2,4-triazole on condensation with chloro-, bromo- and nitro-substituted 2-hydroxybenzaldehyde yields triazole derived Schiff base ligands. It is an efficient electrolyte additive in dye sensitized solar cell.
Application
3-Amino-5-methylthio-1H-1,2,4-triazole was used in the synthesis of heterocyclic disperse azo dyes based on 8-hydroxyquinoline.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Density Functional Theory Study of Additives in Electrolytes of a Dye Sensitized Solar Cell.
Lee M-E, et al.
Bull. Korean Chem. Soc., 34(8), 2491-2491 (2013)
Zahid H Chohan et al.
Journal of enzyme inhibition and medicinal chemistry, 28(5), 944-953 (2012-07-19)
A new series of four biologically active triazole derived Schiff base ligands (L(1)-L(4)) and their cobalt(II), nickel(II), copper(II) and zinc(II) complexes (1-16) have been synthesized and characterized. The ligands were prepared by the condensation reaction of 3-amino-5-methylthio-1H-1,2,4-triazole with chloro-, bromo-
Synthesis and spectroscopic properties of new hetarylazo 8-hydroxyquinolines from some heterocyclic amines.
Saylam A, et al.
Dyes and Pigments, 76(2), 470-476 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service