All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8O
CAS Number:
Molecular Weight:
96.13
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.439 (lit.)
bp
92-93 °C/768 mmHg (lit.)
density
0.912 g/mL at 25 °C (lit.)
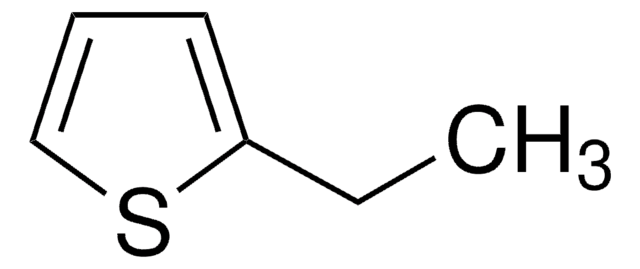
SMILES string
CCc1ccco1
InChI
1S/C6H8O/c1-2-6-4-3-5-7-6/h3-5H,2H2,1H3
InChI key
HLPIHRDZBHXTFJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Ethylfuran undergoes tetraphenylporphin-photosensitized oxygenation in non-polar aprotic solvents via (4+2)-cycloaddition of singlet oxygen to yield the corresponding monomeric unsaturated secondary ozonide.
Application
2-Ethylfuran was used to investigate the gas phase products formed from the Cl atoms initiated reactions of 2-ethylfuran by in situ long-path FTIR absorption spectroscopy. It was also used in the synthesis of 4-oxo-(E)-2-hexenal via ring opening reaction using aqueous N-bromosuccinimide.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
28.4 °F - closed cup
Flash Point(C)
-2 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Singlet oxygen photooxygenation of furans: Isolation and reactions of (4+ 2)-cycloaddition products (unsaturated sec.-ozonides).
Gollnick K and Griesbeck A.
Tetrahedron, 41(11), 2057-2068 (1985)
Atmospheric degradation of alkylfurans with chlorine atoms: Product and mechanistic study.
Villanueva F, et al.
Atmospheric Environment, 43(17), 2804-2813 (2009)
Audrey R Smith et al.
Journal of mass spectrometry : JMS, 50(11), 1206-1213 (2015-10-28)
Absolute photoionization cross sections of the molecules 2-ethylfuran, 2-acetylfuran and furfural, including partial ionization cross sections for the dissociative ionized fragments, are measured for the first time. These measurements are important because they allow fuel quantification via photoionization mass spectrometry
Koji Noge et al.
Journal of chemical ecology, 38(8), 1050-1056 (2012-10-12)
We examined whether shared volatiles found in various heteropteran species and developmental stages function to repel predators. The nymphal dorsal abdominal gland secretions of Riptortus pedestris (Heteroptera: Alydidae) and Thasus acutangulus (Heteroptera: Coreidae), and the metathoracic scent gland secretion of
Francisco J Hidalgo et al.
Journal of agricultural and food chemistry, 62(49), 12045-12051 (2014-11-25)
The carbonyl-scavenging ability of 2-amino-1-methylbenzimidazole (AMBI) and the heterocyclic aromatic amine 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) was investigated in an attempt to identify new routes that can modify the carbonyl content of foods. The reaction of both AMBI and PhIP with 2-alkenals, 2,4-alkadienals
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service