127442
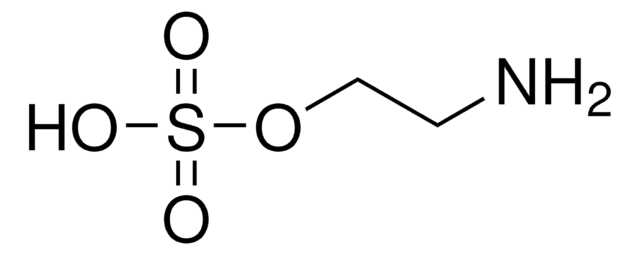
Aminomethanesulfonic acid
97%
Synonym(s):
(Aminomethyl)sulfonic acid, 1-Aminomethanesulfonic acid, Aminomethanesulfonic acid, Aminomethanesulphonic acid, aminomethylsulfonic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
NH2CH2SO3H
CAS Number:
Molecular Weight:
111.12
Beilstein:
1811756
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
184 °C (dec.) (lit.)
functional group
amine
sulfonic acid
SMILES string
NCS(O)(=O)=O
InChI
1S/CH5NO3S/c2-1-6(3,4)5/h1-2H2,(H,3,4,5)
InChI key
OBESRABRARNZJB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Aminomethanesulfonic acid can be used as a reactant to synthesize: 1-Sulfomethyltetrazole-5-thiol disodium salts, which are useful key intermediates for the preparation of cefonicid sodium. Tridentate N-(2-hydroxybenzyl)aminomethane sulfonic acid Schiff-base ligand by reacting with salicylaldehyde.
It can also be used to functionalize the surface of single-walled carbon nanotubes (SWCNT) or porous metal-organic frameworks (MOF) to introduce the sulfonic acid functional groups for the preparation of nanocomposites.
It can also be used to functionalize the surface of single-walled carbon nanotubes (SWCNT) or porous metal-organic frameworks (MOF) to introduce the sulfonic acid functional groups for the preparation of nanocomposites.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Sonoko Ishizaki-Koizumi et al.
Biochemical and biophysical research communications, 322(2), 514-519 (2004-08-25)
The activation of Kupffer cells represents a central mechanism of liver injury involving the production of TNF-alpha. It is known that glycine prevents LPS-induced production of TNF-alpha in isolated Kupffer cells. In this study, the possibility that glycine analogues might
W S Lewis et al.
The Journal of biological chemistry, 266(31), 20823-20827 (1991-11-05)
The composition and structural aspects of the amino and carboxylic acid groups required for incorporation into peptides by transpeptidation and inhibition of hydrolysis in carboxypeptidase Y-catalyzed reactions were studied. Separation of these two groups by even one carbon prevents incorporation
Copper (II) complexes of Schiff-base and reduced Schiff-base ligands: Influence of weakly coordinating sulfonate groups on the structure and oxidation of 3, 5-DTBC
Sreenivasulu B, et al.
European Journal of Inorganic Chemistry, 2005(22), 4635-4645 (2005)
Effect of single-walled carbon nanotubes on the transport properties of sulfonated poly (styrene-isobutylene-styrene) membranes
Aviles-Barreto SL and Suleiman D
Journal of Membrane Science , 474, 92-102 (2015)
Adsorptive denitrogenation of model fuels with porous metal-organic frameworks (MOFs): Effect of acidity and basicity of MOFs
Ahmed I, et al.
Applied Catalysis. B, Environmental, 129, 123-129 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service