T7915
Thioredoxin Reductase from Escherichia coli
ammonium sulfate suspension, >25 units/mg protein (Bradford)
Synonym(s):
NADPH:oxidized thioredoxin oxidoreductase
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
biological source
Escherichia coli
Quality Level
form
ammonium sulfate suspension
specific activity
>25 units/mg protein (Bradford)
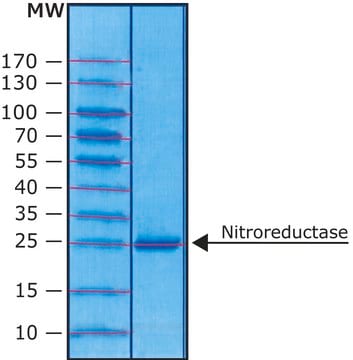
mol wt
70 kDa
technique(s)
activity assay: suitable
UniProt accession no.
storage temp.
2-8°C
Gene Information
Escherichia coli K12 ... trxB(949054)
General description
Research area: Cell Signaling
Thioredoxin reductases (TrxRs) belongs to family of selenium-containing pyridine nucleotide-disulphide oxidoreductases.
Thioredoxin reductases (TrxRs) belongs to family of selenium-containing pyridine nucleotide-disulphide oxidoreductases.
Application
Thioredoxin Reductase from Escherichia coli can be used in peroxidase-coupled thioredoxin system assay for assessing the peroxidase activitiy of Cys-based thiol peroxidases.Thioredoxin Reductase from Escherichia coli has been used:
- for determining the enzymatic activity of His6-Ahp1p.
- to assay C. elegans TRXR using the TXN-dependent activity assay.
- to investigate the biological reducing systems for organic hydroperoxide resistance R gene (OhrR).
- for enzymatic targeting of auranofin to test its antimicrobial properties.
- in thioredoxin reductase activity and peroxiredoxin assay.
- to study the synergy between broccoli sprout extract and selenium in the upregulation of thioredoxin reductase in human hepatocytes.
Thioredoxin reductase from Escherichia coli has been used in thioredoxin reductase activity and peroxiredoxin assay.
It has also been used to study the synergy between broccoli sprout extract and selenium in the upregulation of thioredoxin reductase in human hepatocytes.
It has also been used to study the synergy between broccoli sprout extract and selenium in the upregulation of thioredoxin reductase in human hepatocytes.
Biochem/physiol Actions
An FAD-containing enzyme involved in the transfer of hydrogen from E. coli thioredoxin to other proteins thus providing a powerful disulfide reductase system.
Thioredoxin reductase is a FAD containing enzyme, which transfers the reducing equivalent from NADPH to the disulphide bond of the enzyme by using FAD moiety within the Cys-Ala-Thr-Cys sequence. It can also reduce Trx-S2 to Trx-(SH)2 by using NADPH.
Thioredoxin reductase (TrxR) is an NADPH-dependent oxidoreductase containing one FAD per subunit that reduces the active site disulfide in oxidised thioredoxin (Trx). The molecular weight of the isozymes from mammalian sources vary between 55-67 kDa as compared with 35 kDa in prokaryotes, plants or yeast. The substrate specificity of the mammalian enzyme is much broader than the prokaryotic enzyme reducing both mammalian and E. coli thioredoxins as well as well as non-disulfide substrates such selenite, lipoic acids, lipid hydroperoxides and hydrogen peroxide.
Unit Definition
One unit will cause an increase in absorbance of 1.0 at 412 nm (when measured in a coupled assay with E. coli thioredoxin and DTNB) per min per mL at pH 7.0 at 25 °C.
Physical form
Suspension in 3.6 M (NH4)2SO4 containing 30 mM potassium phosphate buffer, pH 7.5, and 2 mM EDTA.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Deletion of Thioredoxin Reductase and Effects of Selenite and Selenate Toxicity in Caenorhabditis elegans
Boehler CJ, et al.
PLoS ONE, 8(8), e71525-e71525 (2013)
Synergy between broccoli sprout extract and selenium in the upregulation of thioredoxin reductase in human hepatocytes
Li D, et al.
Food Chemistry, 110(1), 193-198 (2008)
NR1D1 Recruitment to Sites of DNA Damage Inhibits Repair and Is Associated with Chemosensitivity of Breast Cancer
Ka NL, et al.
Journal of Separation Science, 77(9), 2453?2463-2453?2463 (2017)
Purification and characterization of ferredoxin-NAD (P)+ reductase from the green sulfur bacterium Chlorobium tepidum
Seo D, et al.
Biochim. Biophys. Acta Gen. Subj., 1597(1), 123-132 (2002)
Proteolysis-targeting chimeras: A promising technique in cancer therapy for gaining insights into tumor development
Lv M, et al.
Cancer Letters, 539, 215716-215716 (2022)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service