All Photos(1)
About This Item
Empirical Formula (Hill Notation):
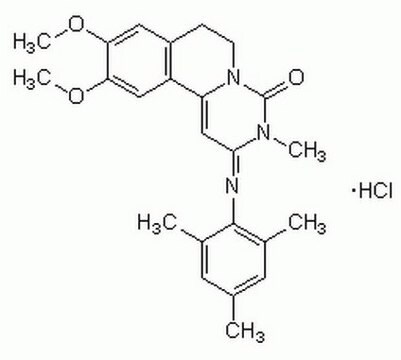
C24H27N3O3 · HCl
CAS Number:
Molecular Weight:
441.95
MDL number:
UNSPSC Code:
41106305
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Assay
≥98%
form
powder
SMILES string
Cl[H].COc1cc2CCN3C(=O)N(C)C(\C=C3c2cc1OC)=N\c4c(C)cc(C)cc4C
InChI
1S/C24H27N3O3.ClH/c1-14-9-15(2)23(16(3)10-14)25-22-13-19-18-12-21(30-6)20(29-5)11-17(18)7-8-27(19)24(28)26(22)4;/h9-13H,7-8H2,1-6H3;1H/b25-22+;
InChI key
DTCZZBVPTHVXFA-OSMRDGEFSA-N
Gene Information
human ... PDE3A(5139) , PDE3B(5140)
Application
Trequinsin has been used as a PDE3 inhibitor in rat juxtaglomerular cells. This study reported that trequinsin can enhance cellular cAMP content, forskolin-induced cAMP synthesis, and renin release in cells.
Biochem/physiol Actions
Phosphodiesterase III inhibitor.
Trequinsin is a strong antihypertensive agent that has a hemodynamic profile similar to that of arteriolar dilators. Trequinsin can block platelet aggregation and also inhibit tissue factor expression in human endothelial cells,.
Features and Benefits
This compound is a featured product for Cyclic Nucleotide research. Click here to discover more featured Cyclic Nucleotide products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Phosphodiesterases page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
R F Booth et al.
Biochemical pharmacology, 36(20), 3517-3521 (1987-10-15)
The discovery and structure-activity of a new class of renal artery phosphodiesterase inhibitors is reported, some of which are highly selective for the guanosine cyclic 3',5'-monophosphate phosphodiesterase. One of these compounds, 5,6-dihydro-8,9,11,12-tetramethoxy-1,3-dioxo-1H-benz[f]- isoquino [8,1,2- hij]quinazoline-2(3H)-carboxylic acid, ethyl ester (9), is
R A Rius et al.
Life sciences, 54(22), 1735-1743 (1994-01-01)
In primary cultured bovine adrenal chromaffin cells (BACC), pituitary adenylate cyclase activating polypeptide 1-38 (PACAP) produced a dose related increase in tyrosine hydroxylase (TH) Vmax when measured 48 hours after the beginning of the treatment; a significant increase was observed
R Pillai et al.
The Journal of biological chemistry, 269(48), 30676-30681 (1994-12-02)
We have created a series of deletion mutants of a human cardiac cAMP phosphodiesterase in order to define sequences necessary for function and to identify residues required for inhibition by cGMP and by the drugs milrinone and trequinsin. These truncated
Jorge E Torres-López et al.
European journal of pharmacology, 519(1-2), 75-79 (2005-08-23)
The local peripheral (subcutaneous) injection of phosphodiesterase 3 inhibitor trequinsin dose-dependently enhanced formalin-evoked flinching during late second phase of this test. Treatment with the nitric oxide synthase inhibitor N-L-nitro-arginine methyl ester or guanylyl cyclase inhibitor 1-H-[1,2,4,]oxadiazolo[4,3-a]quinoxalin-1-one significantly reversed trequinsin-induced pronociceptive
Ulla G Friis et al.
Circulation research, 90(9), 996-1003 (2002-05-23)
We tested the hypothesis that cGMP stimulates renin release through inhibition of the cAMP-specific phosphodiesterase 3 (PDE3) in isolated rat juxtaglomerular (JG) cells. In addition, we assessed the involvement of PDE4 in JG-cell function. JG cells expressed PDE3A and PDE3B
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service