G8162
β-Glucuronidase from Escherichia coli
aqueous glycerol solution, ≥5,000,000 units/g protein, pH 6.8 (biuret)
Synonym(s):
β-D-Glucuronide glucuronosohydrolase
About This Item
Recommended Products
biological source
Escherichia coli
form
aqueous glycerol solution
specific activity
≥5,000,000 units/g protein, pH 6.8 (biuret)
mol wt
69-71 kDa
shipped in
wet ice
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
Application
The optimal conditions for the enzymatic hydrolysis of α-hydroxytriazolam, one of the major metabolites of triazolam in human urine, were determined using β-glucuronidase Type IX-A.
It is used as a reporter gene in GUS assays to monitor gene expression.
Learn more
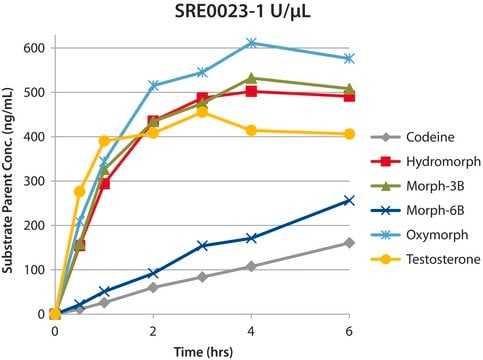
about recent application data generated by Sigma R&D to optimize hydrolysis for different drug classes using enzymes from different sources and the use of a chromatographicaly purified enzyme to reduce the effect of esterase activity resulting in conversion of 6-MAM to Morphine
Biochem/physiol Actions
Unit Definition
Physical form
comparable product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service