D4681
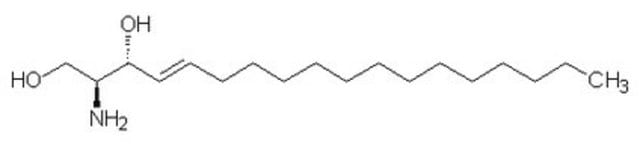
L-threo-Dihydrosphingosine
≥95% (TLC)
Synonym(s):
Safingol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C18H39NO2
CAS Number:
Molecular Weight:
301.51
MDL number:
UNSPSC Code:
12352211
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (TLC)
form
powder
storage temp.
−20°C
SMILES string
CCCCCCCCCCCCCCC[C@H](O)[C@@H](N)CO
InChI
1S/C18H39NO2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-18(21)17(19)16-20/h17-18,20-21H,2-16,19H2,1H3/t17-,18-/m0/s1
InChI key
OTKJDMGTUTTYMP-ROUUACIJSA-N
Biochem/physiol Actions
Sphingosine kinase inhibitor; protein kinase C alpha (PKCα) -specific inhibitor; Sphingosine analog; potentiates the effect of doxorubicin (DOX) in tumor-bearing animals.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J W Darges et al.
Advances in experimental medicine and biology, 400A, 387-392 (1997-01-01)
The sphingosine analog L-threo-dihydrosphingosine has been shown to inhibit protein kinase C (PKC) isoenzymes in mixed micelle and vesicle assays. This compound also inhibited the reactive oxygen intermediates (ROI) released from isolated neutrophils (IC50 approximately 2 microM) and phorbol ester-induced
G K Schwartz et al.
Clinical cancer research : an official journal of the American Association for Cancer Research, 3(4), 537-543 (1997-04-01)
We performed a pilot clinical trial with safingol (L-threo-dihydrosphingosine), a protein kinase C-specific inhibitor that potentiates the effect of doxorubicin (DOX) in tumor-bearing animals. Safingol was initially administered as a 1-h infusion at escalating doses. Fourteen days later, patients received
Yi-Hsin Hsu et al.
Cancer research, 74(17), 4822-4835 (2014-06-28)
Triple-negative breast cancer (TNBC) is a highly heterogeneous and recurrent subtype of breast cancer that lacks an effective targeted therapy. To identify candidate therapeutic targets, we profiled global gene expression in TNBC and breast tumor-initiating cells with a patient survival
Avraham Ashkenazi et al.
FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 26(11), 4628-4636 (2012-08-09)
Understanding the structural organization of lipids in the cell and viral membranes is essential for elucidating mechanisms of viral fusion that lead to entry of enveloped viruses into their host cells. The HIV lipidome shows a remarkable enrichment in dihydrosphingomyelin
Hyun Joon Kim et al.
Journal of lipid research, 53(8), 1701-1707 (2012-06-05)
The sphingolipids are a diverse family of lipids with important roles in membrane compartmentalization, intracellular signaling, and cell-cell recognition. The central sphingolipid metabolite is ceramide, formed by the transfer of a variable length fatty acid from coenzyme A to a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service