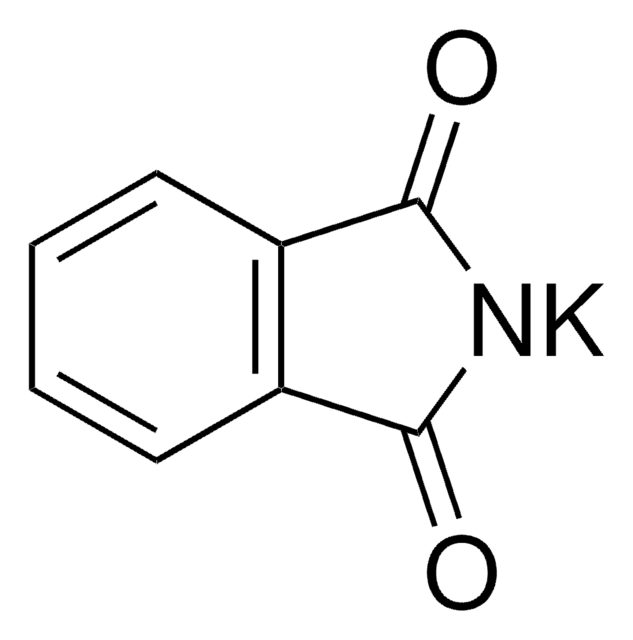
79790
Phthalimide potassium salt
purum, ≥99.0% (NT)
Synonym(s):
PPI, 1,3-Dihydro-1,3-dioxoisoindole potassium salt, Potassium phthalimide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H4KNO2
CAS Number:
Molecular Weight:
185.22
Beilstein:
3598719
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
purum
Quality Level
Assay
≥99.0% (NT)
form
powder
impurities
≤0.5% water
mp
>300 °C (lit.)
SMILES string
[K]N1C(=O)c2ccccc2C1=O
InChI
1S/C8H5NO2.K/c10-7-5-3-1-2-4-6(5)8(11)9-7;/h1-4H,(H,9,10,11);/q;+1/p-1
InChI key
FYRHIOVKTDQVFC-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Phthalimide potassium salt (PPI) is widely used for the synthesis of primary amines from corresponding alkyl halides, known as Gabriel synthesis. Some of the other applications are:
- Preparation of phthalimidogold precatalyst for gold catalysis.
- It can be used in the palladium-catalyzed enantioselective synthesis of α- and β-amino acids.
- Potassium phthalimide is as an effective organocatalyst for the cyanosilylation of carbonyl compounds to synthesize cyanohydrin trimethylsilyl ethers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
346.3 °F - closed cup
Flash Point(C)
174.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
One-pot synthesis of core-expanded naphthalene diimides: enabling N-substituent modulation for diverse n-type organic materials.
Hu Y, et al.
Organic Letters, 14(1), 292-295 (2011)
γ?Aminobutyric Acid
DeWitt C C
Organic Syntheses, 4-4 (1943)
A facile one-pot synthesis of N-substituted phthalimides using a catalytic amount of crown ether.
Soai K, et al.
Bulletin of the Chemical Society of Japan, 55(5), 1671-1672 (1982)
Activation of trimethylsilyl cyanide by potassium phthalimide for facile synthesis of TMS-protected cyanohydrins.
Dekamin MG and Karimi Z.
Journal of Organometallic Chemistry, 694(12), 1789-1794 (2009)
Palladium-catalysed asymmetric allylic substitution: synthesis of α-and β-amino acids.
Bower J F, et al.
Journal of the Chemical Society. Perkin Transactions 1, 9, 1411-1420 (1997)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service