67954
4-(Dimethylamino)benzoyl chloride
for HPLC derivatization, LiChropur™, ≥99.0% (HPLC)
Synonym(s):
DMABC
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2NC6H4COCl
CAS Number:
Molecular Weight:
183.63
MDL number:
UNSPSC Code:
41121800
PubChem Substance ID:
NACRES:
NB.21
Recommended Products
grade
for HPLC derivatization
Quality Level
Assay
≥99.0% (HPLC)
99.0-101.0% (AT)
quality
LiChropur™
technique(s)
HPLC: suitable
mp
145-149 °C (lit.)
146-150 °C
suitability
complies for LC-MS
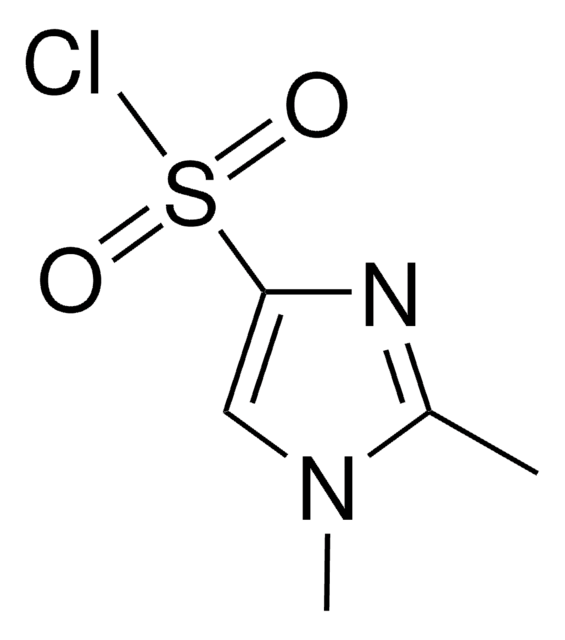
SMILES string
CN(C)c1ccc(cc1)C(Cl)=O
InChI
1S/C9H10ClNO/c1-11(2)8-5-3-7(4-6-8)9(10)12/h3-6H,1-2H3
InChI key
UGJDXRVQCYBXAJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-(Dimethylamino)benzoyl chloride is an organic chemical, basically used as HPLC derivatization reagent.
Material is reported to be very moisture sensitive. Handle under an inert atmosphere
Application
4-(Dimethylamino)benzoyl chloride dissovled in N-methyl-2-pyrrolidinone (NMP) and pyridine may be used in the synthesis of 6-O-(4-(Dimethylamino)benzoyl)curdlan (DABz-Cur) by adding curdlan to dry NMP.
Legal Information
LiChropur is a trademark of Merck KGaA, Darmstadt, Germany
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gaku Fukuhara et al.
Chemical communications (Cambridge, England), 46(48), 9128-9130 (2010-11-10)
A newly synthesized chromophore-modified curdlan functions as a saccharide chemosensor in aqueous solution, enabling us to discriminate tetrasaccharide acarbose from 24 mono-, di-, tri-, and tetrasaccharides.
Yoshio Suzuki et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 20(3), 475-482 (2004-04-08)
Novel labeling reagents, called MS probes, which possess a positively charged quaternary amine moiety and can transform a neutral analyte into a charged compound by simply mixing with the analyte and allowing the mixture to stand from several minutes to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service