8.52418
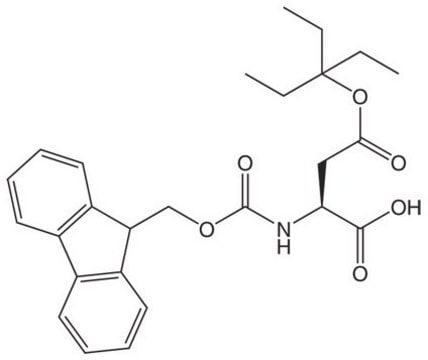
Fmoc-Asp(OBno)-OH
for peptide synthesis, Novabiochem®
Synonym(s):
Fmoc-Asp(OBno)-OH
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C32H43NO6
Molecular Weight:
537.69
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
product name
Fmoc-Asp(OBno)-OH, Novabiochem®
Quality Level
product line
Novabiochem®
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
carboxylic acid
storage temp.
−20°C (−15°C to −25°C)
General description
An excellent derivative for minimizing aspartimide formation during Fmoc SPPS, including those containing the Asp-Gly sequence. The bulky OBno protecting group offers considerably more protection against the formation of aspartimide-related by-products than the commonly used OtBu and OMpe group.,
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aspartimide Formation in Fmoc SPPS
Literature references:
[1] R. Behrendt, et al. (2015) J. Pept. Sci., 21, 680.
[2] R. Behrendt, et al. (2016) J. Pept. Sci., 22, 92.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Overcoming Aspartimide Formation in Fmoc SPPS
Literature references:
[1] R. Behrendt, et al. (2015) J. Pept. Sci., 21, 680.
[2] R. Behrendt, et al. (2016) J. Pept. Sci., 22, 92.
Application
Recently, Fmoc-Asp(OBno)-OH has been used in the synthesis of the mini-protein Omomyc, which has been shown to repress MYC-dependent gene transcription.,
Analysis Note
Color (visual): white to beige
Appearance of substance (visual): powder, chunks or crystals
Identity (IR): passes test
Purity (TLC (018A)): ≥ 95 %
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 97.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Appearance of substance (visual): powder, chunks or crystals
Identity (IR): passes test
Purity (TLC (018A)): ≥ 95 %
Enantiomeric purity: ≥ 99.5 % (a/a)
Assay (HPLC, area%): ≥ 97.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Multiple Synthetic Routes to the Mini-Protein Omomyc and Coiled-Coil Domain Truncations
Brown ZZ, et al.
The Journal of Organic Chemistry, 1466-1466 (2020)
Preventing aspartimide formation in Fmoc SPPS of Asp-Gly containing peptides?practical aspects of new trialkylcarbinol based protecting groups
R. Behrendt, et al.
Journal of Peptide Science, 22, 92-92 (2016)
New t-butyl based aspartate protecting groups preventing aspartimide formation in Fmoc SPPS
R. Behrendt, et al.
Journal of Peptide Science, 21, 680-680 (2015)
Synthesis and evaluation of a multifunctional probe with a high affinity for prostate-specific membrane antigen (PSMA) and bone
Hirata S, et al.
Nuclear Medicine and Biology, 114-115, 34-41 (2022)
Omomyc Reveals New Mechanisms To Inhibit the MYC Oncogene
Mark J. Demma, et al.
Molecular and Cellular Biology, 39 (2019)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service